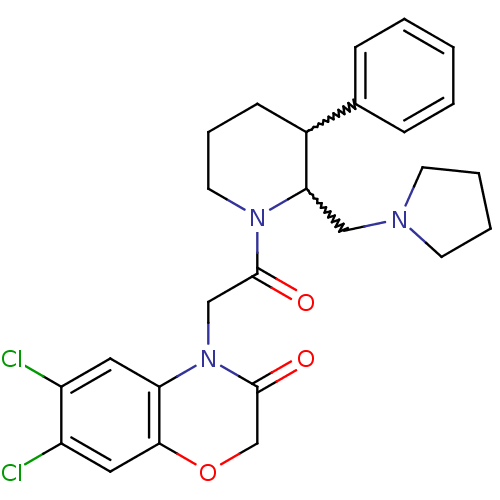
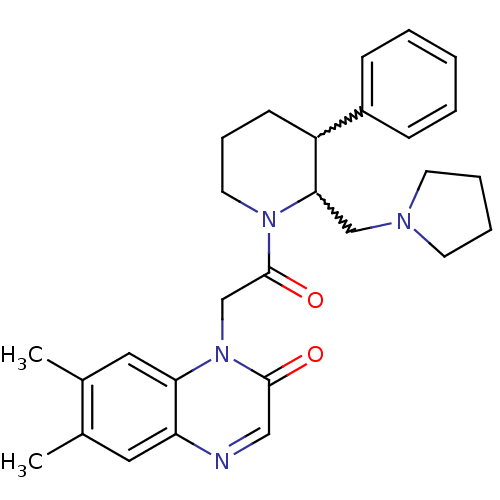
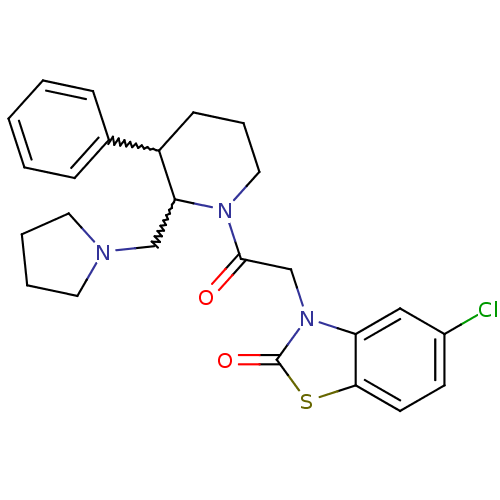
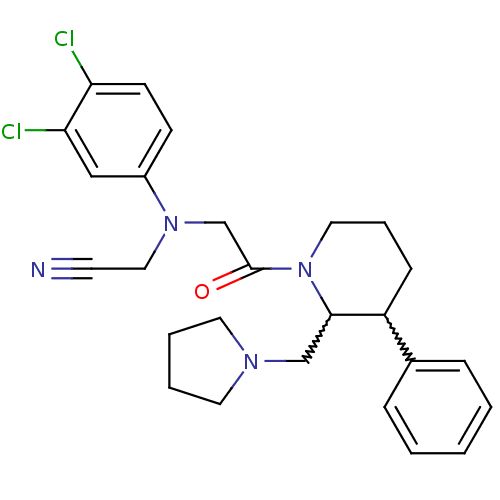
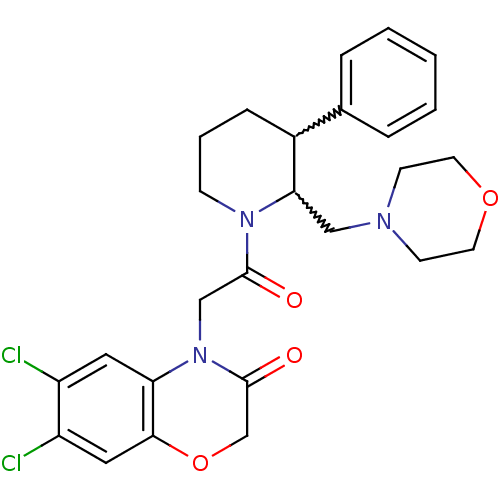
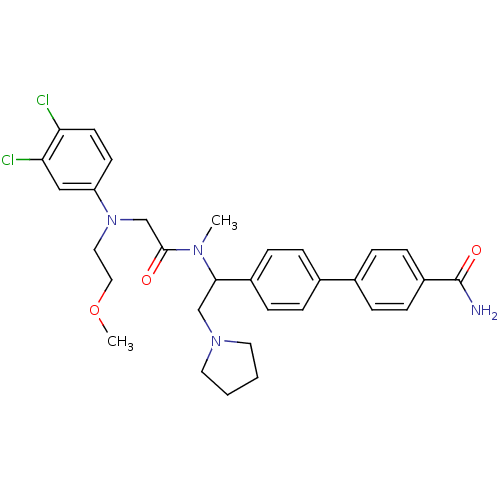
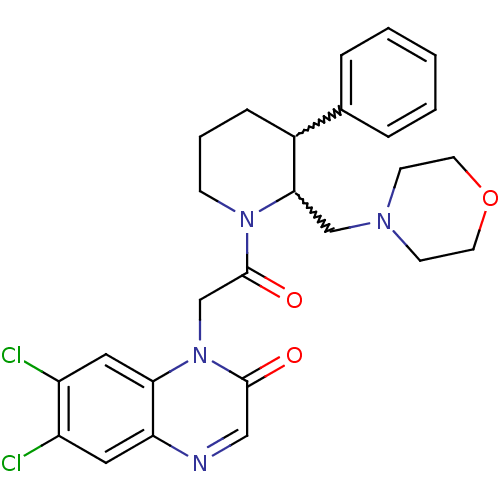
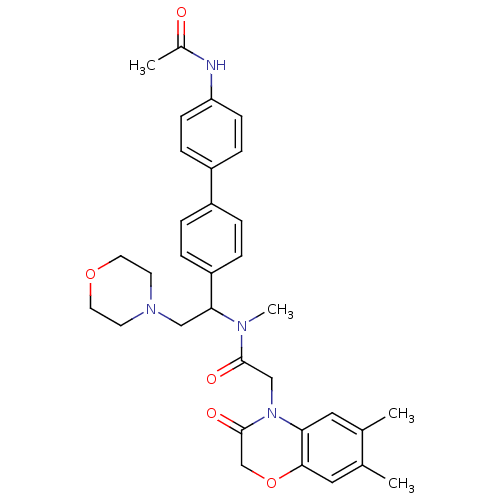
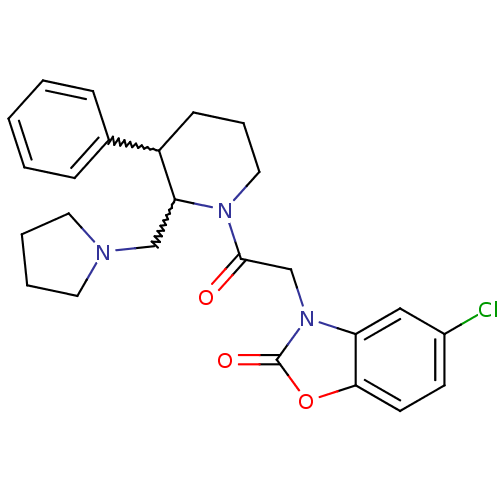
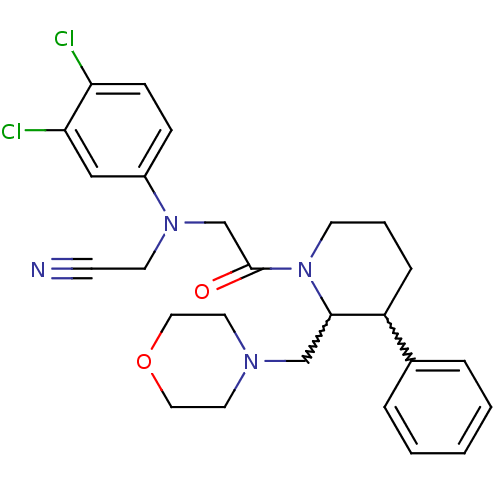
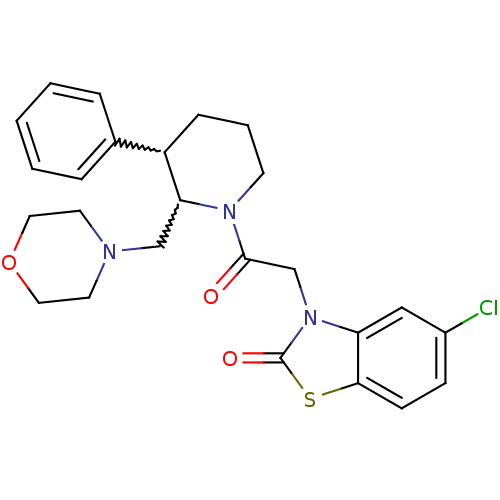
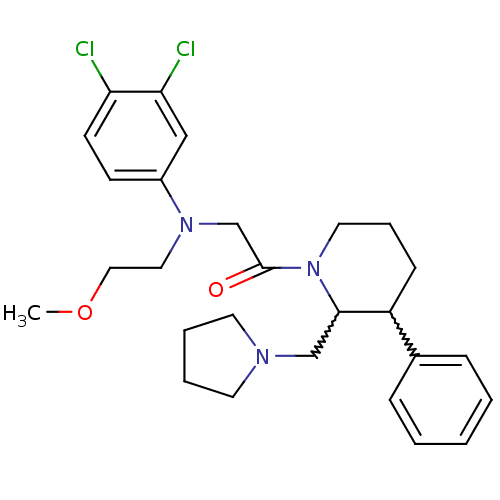
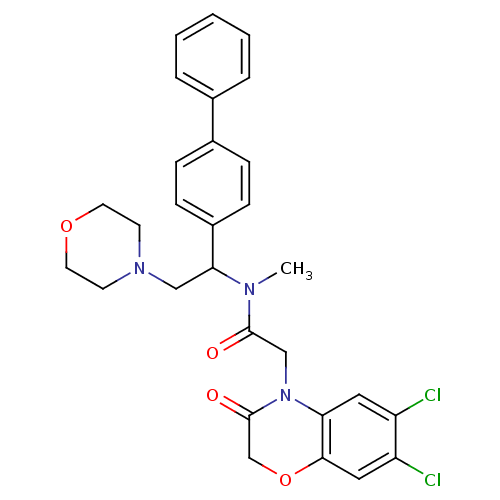
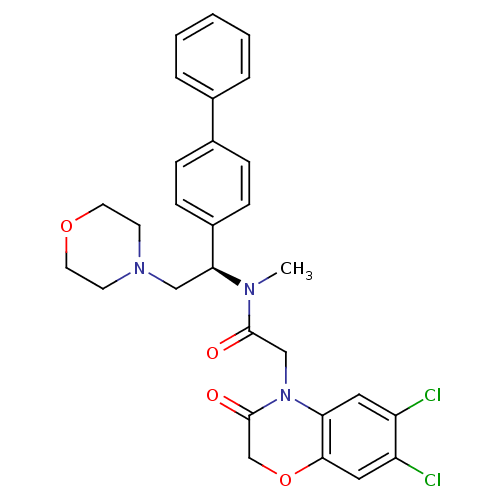
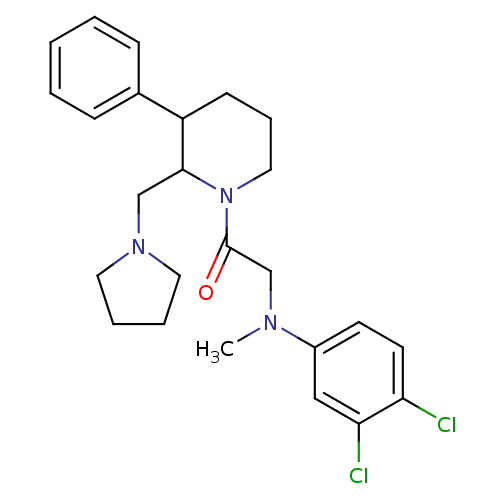
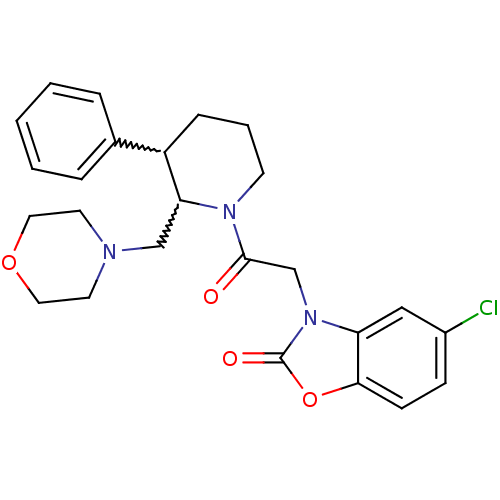
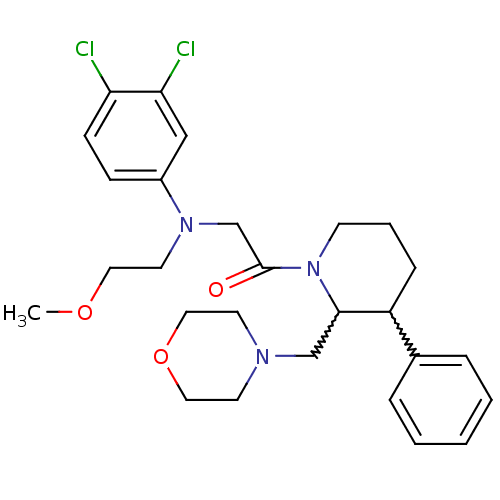
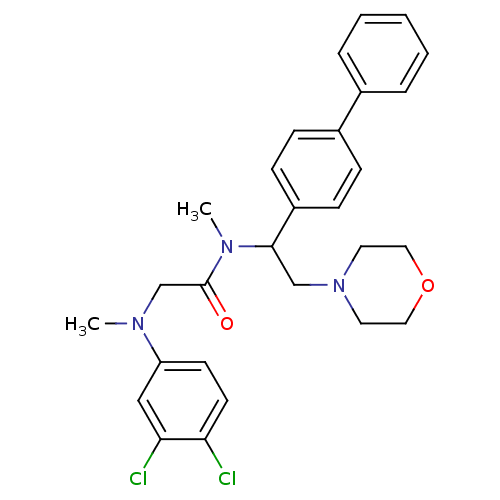
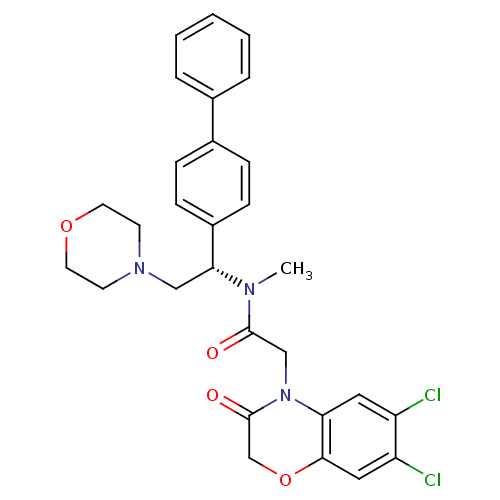
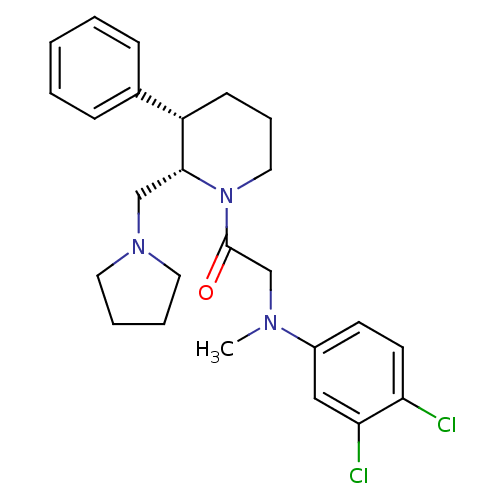
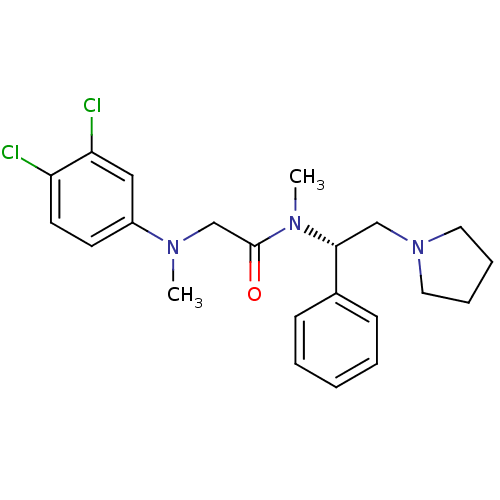
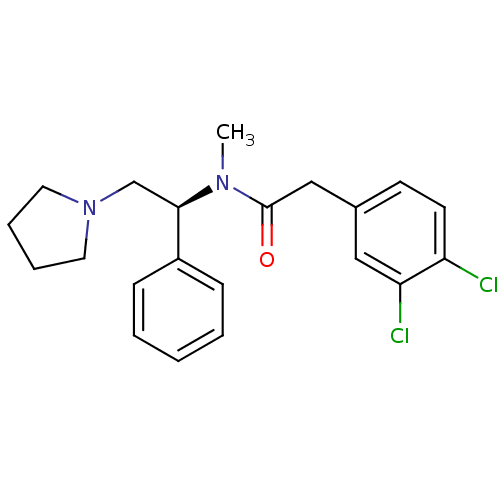
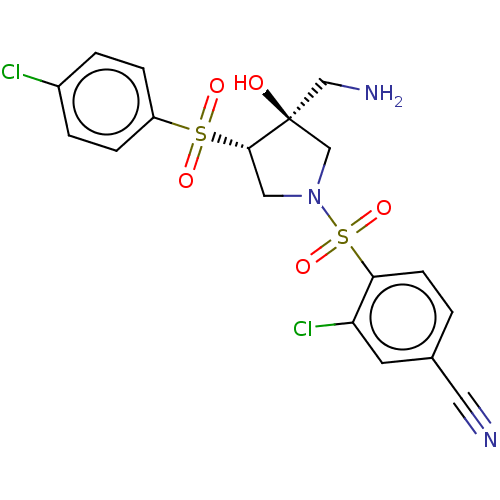
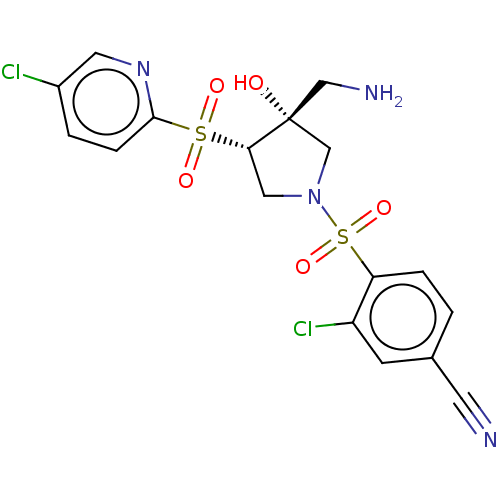
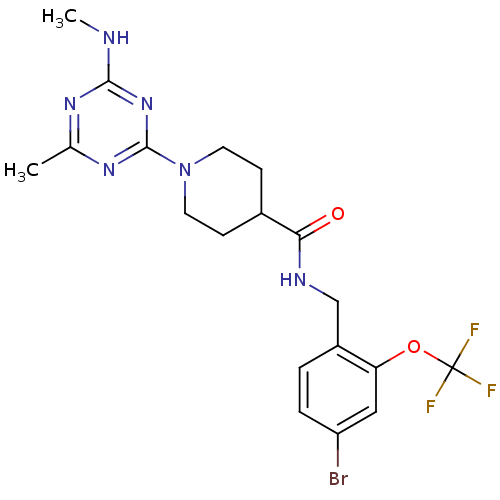
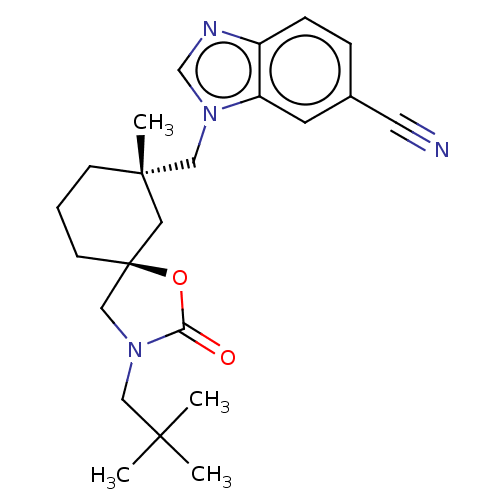
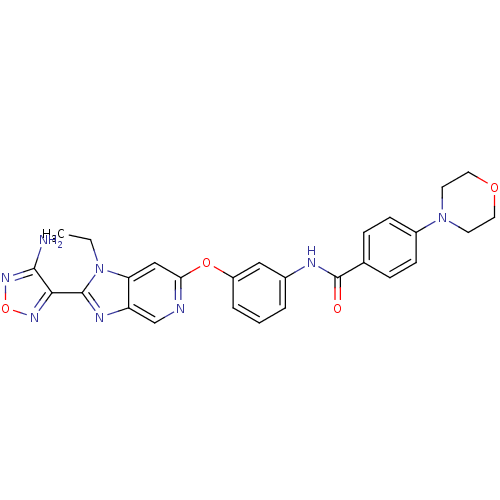
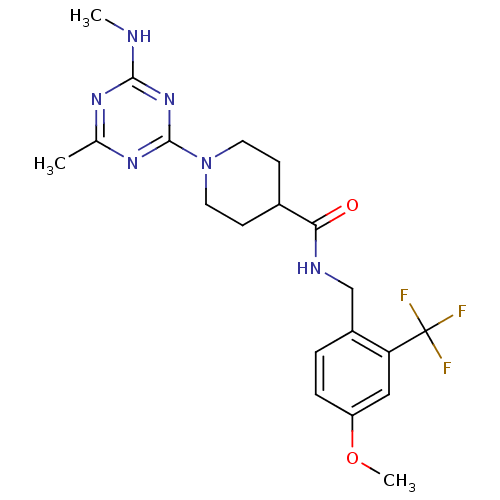
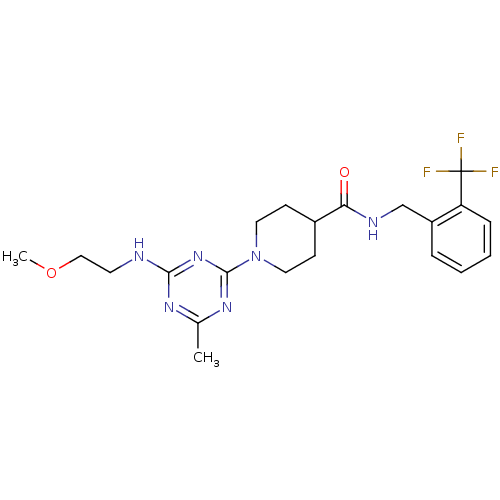
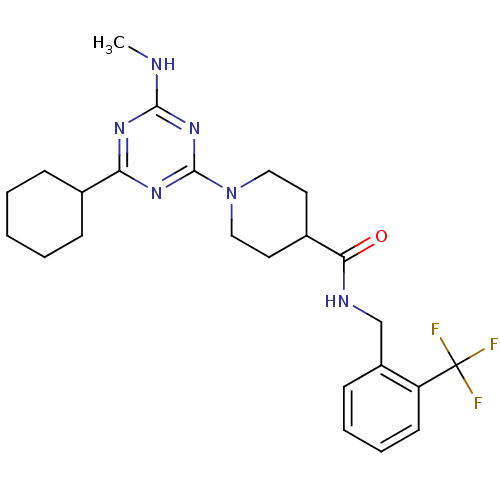
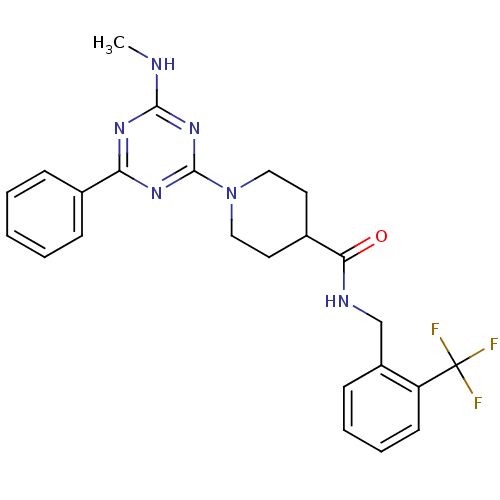
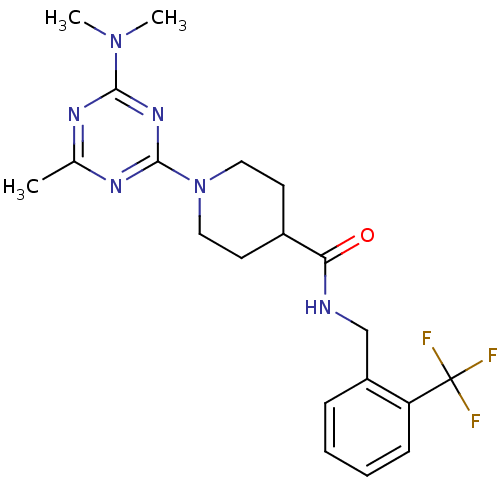
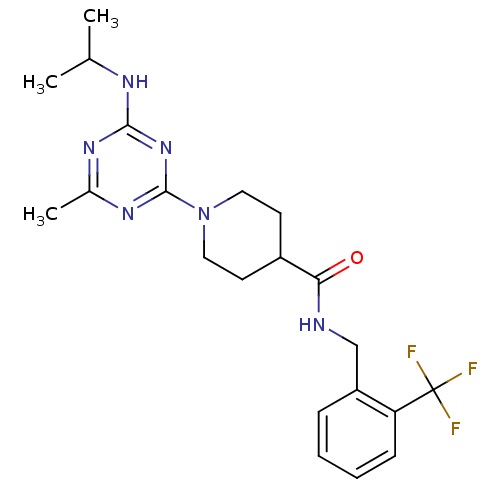
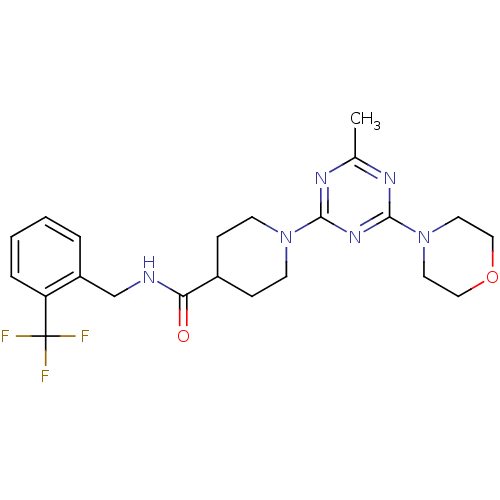
Affinity DataKi: 0.300nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMpH: 7.4 T: 2°CAssay Description:The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Rattus norvegicus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Antagonist activity at rat TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair