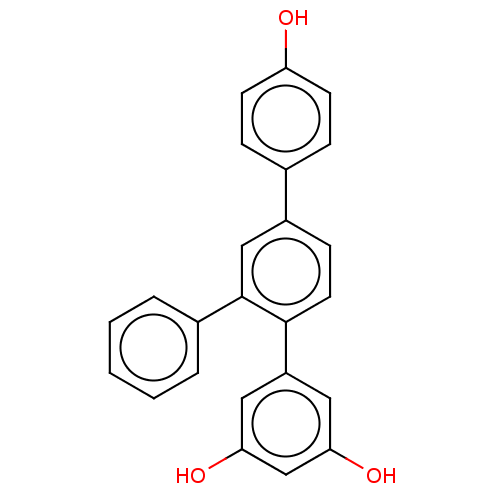
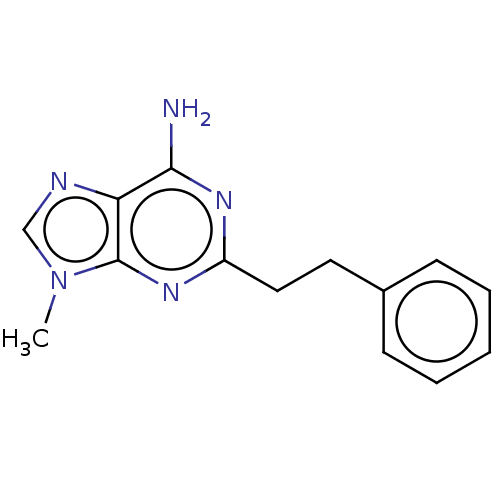
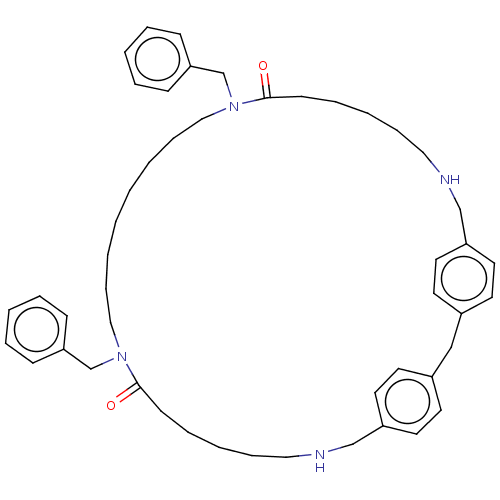
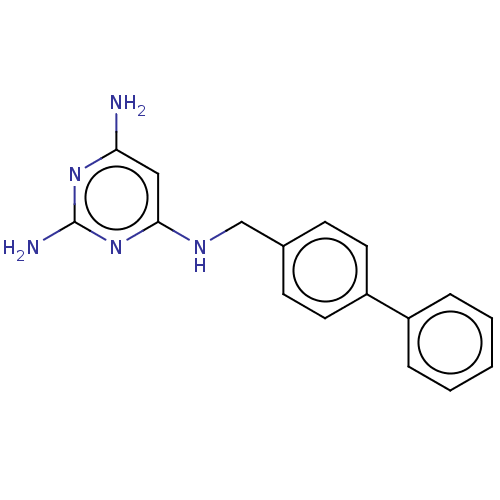
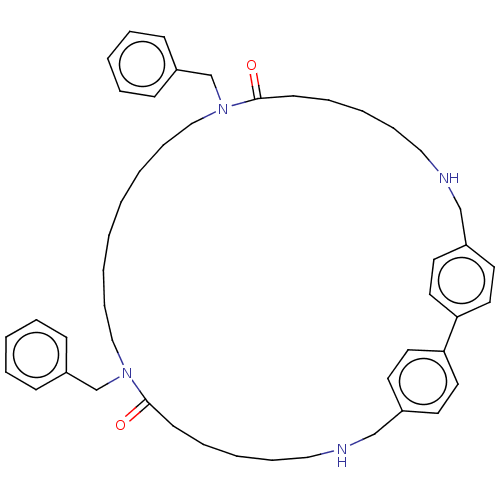
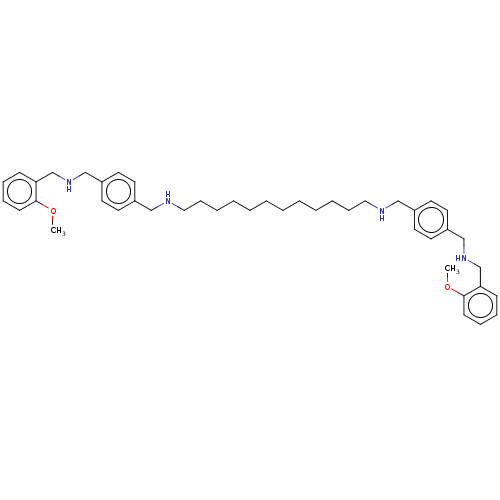
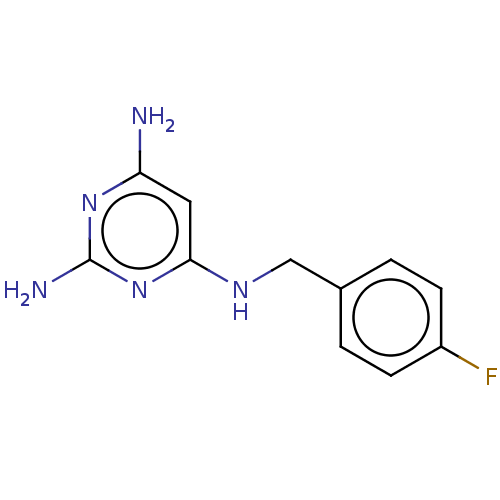
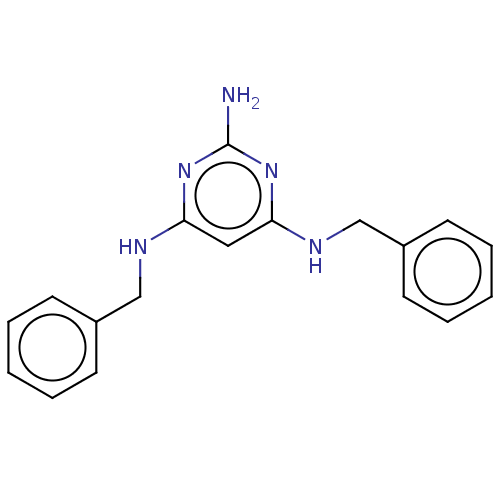
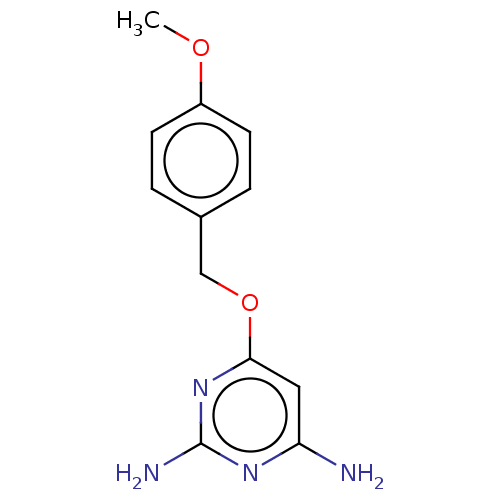
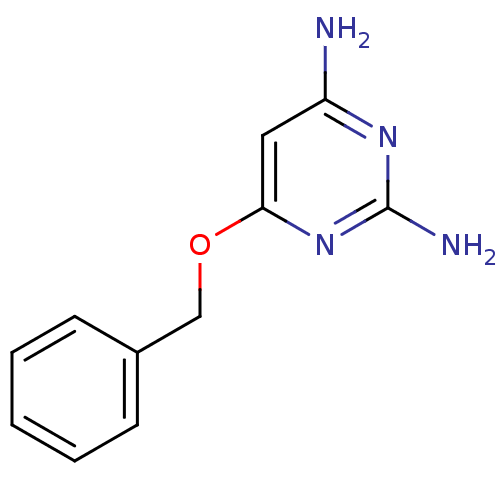
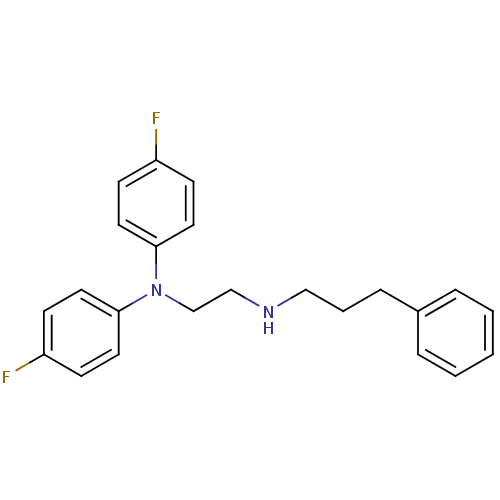
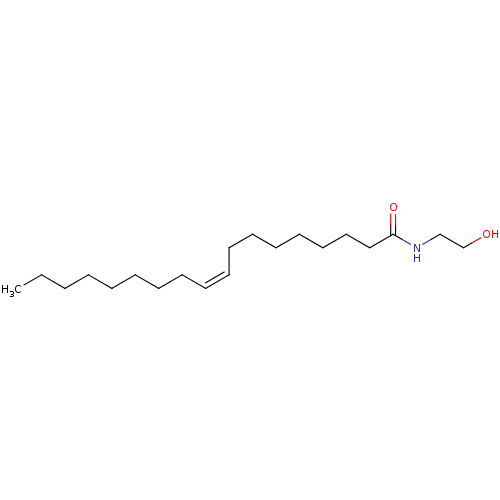
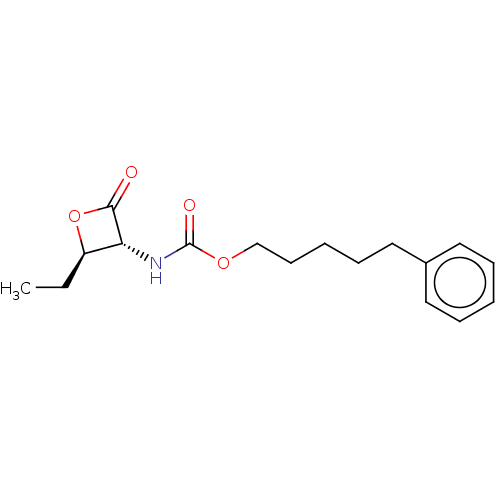
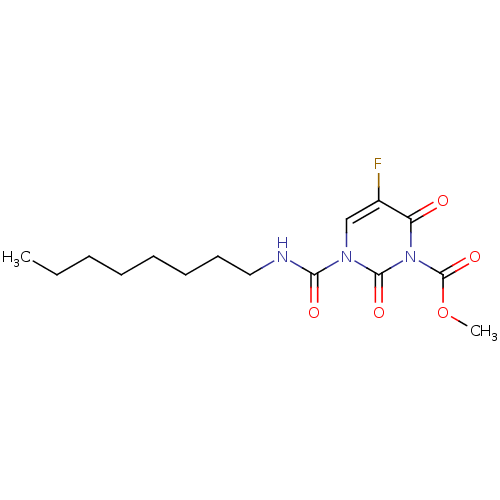
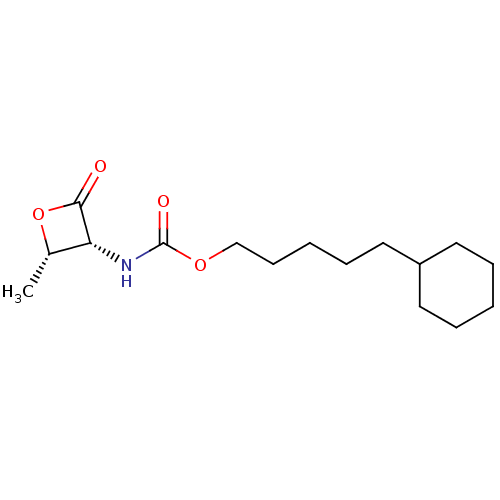
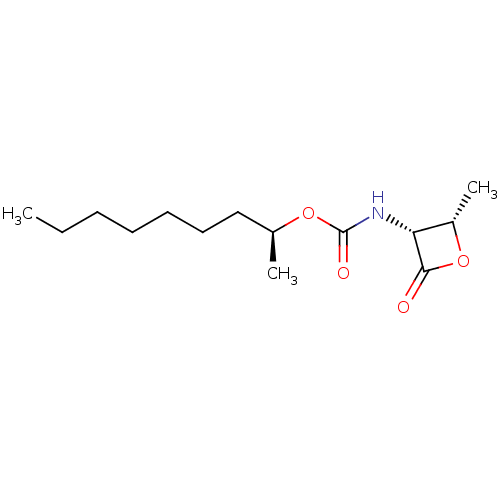
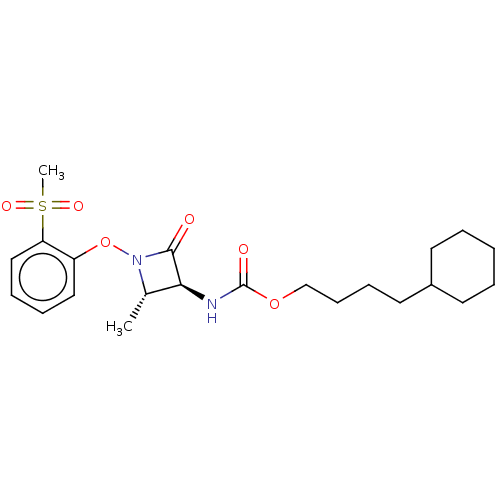
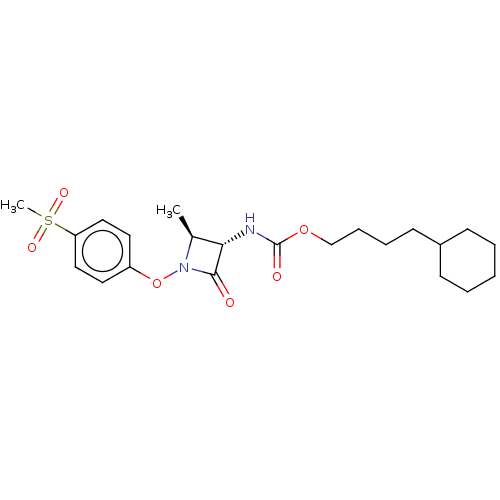
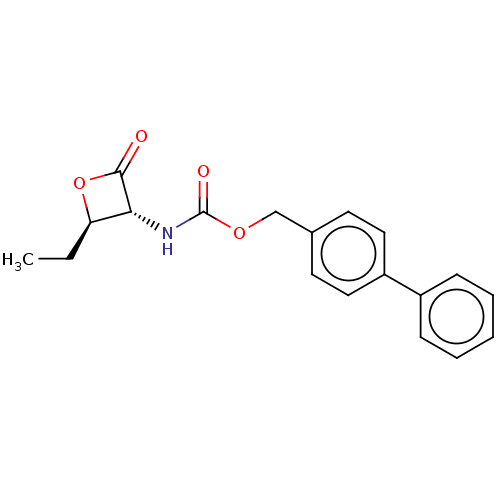
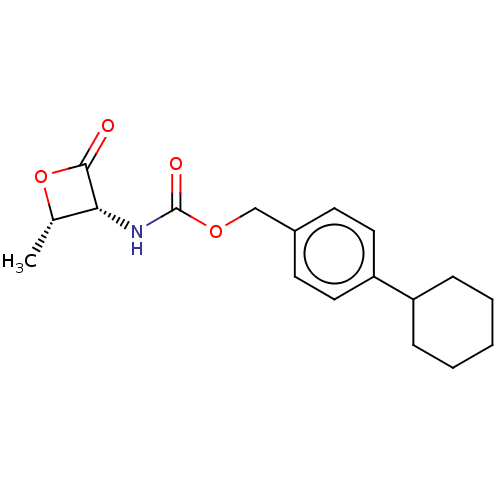
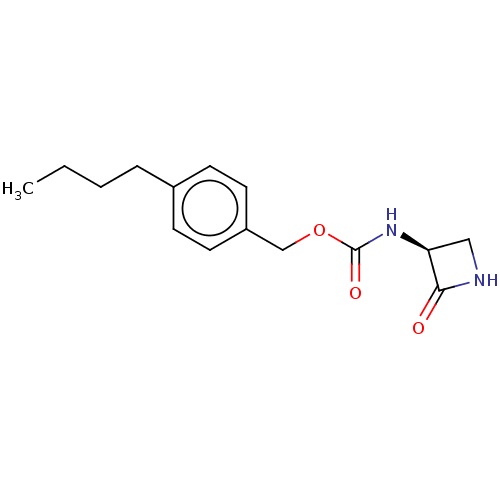
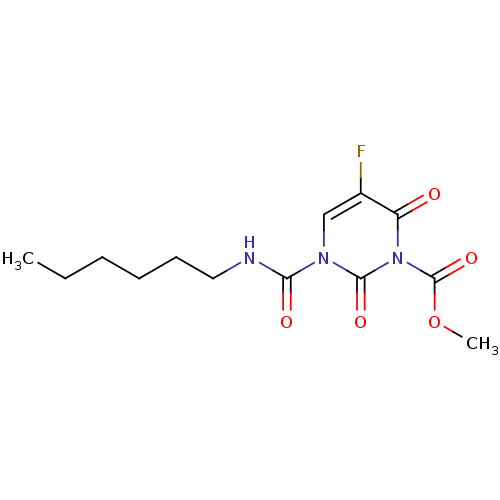
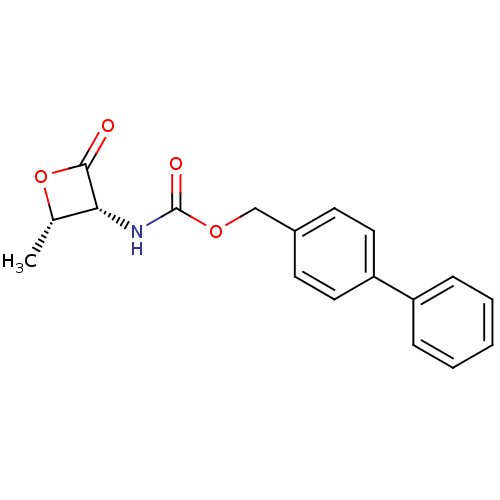
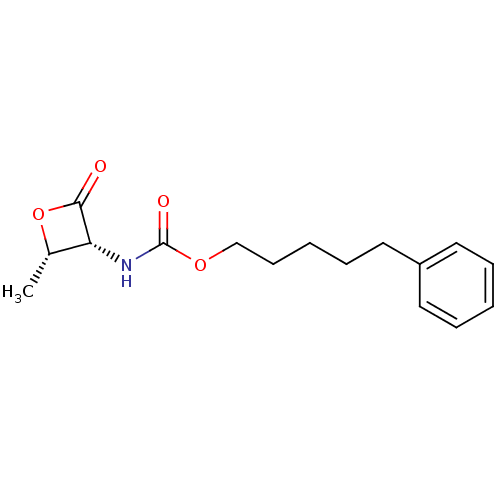
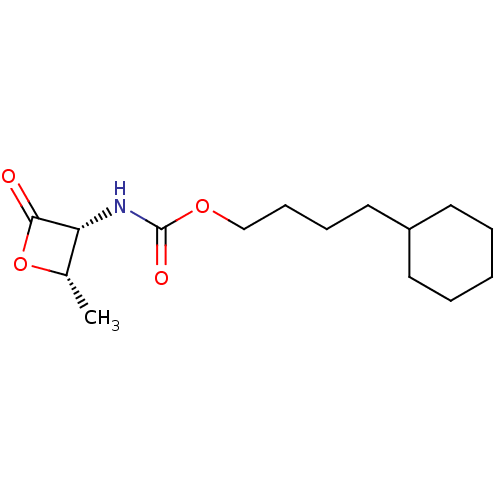
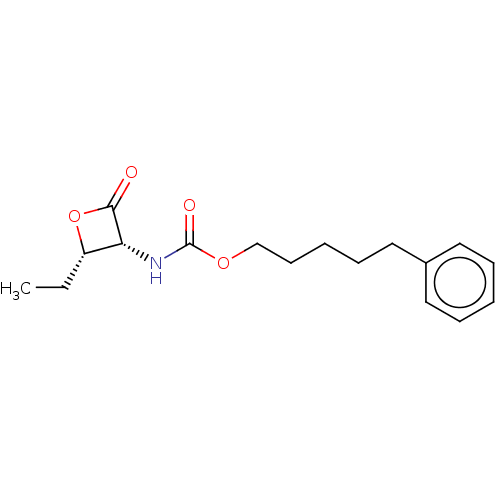
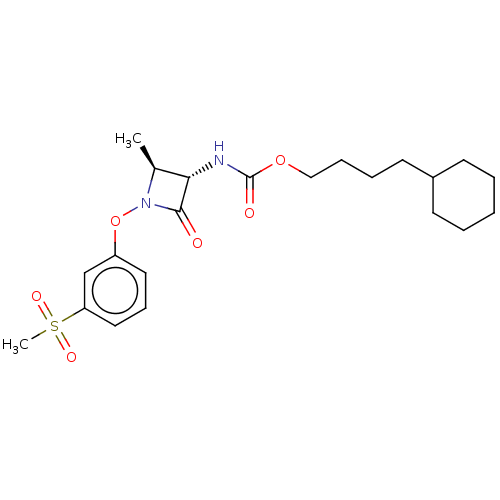
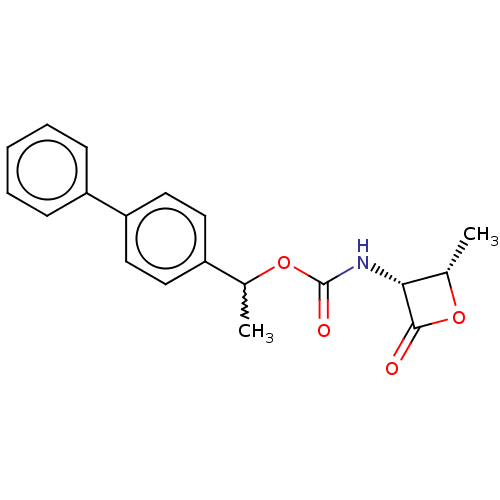
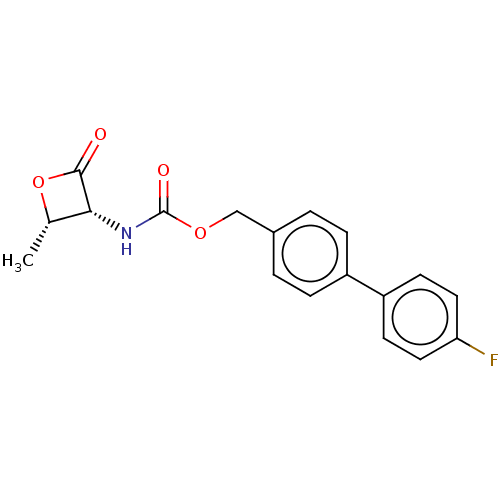
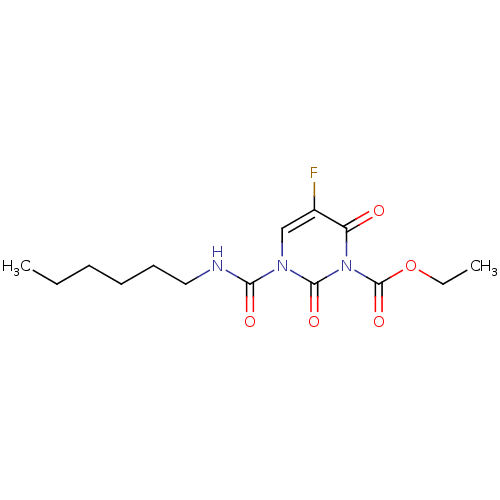
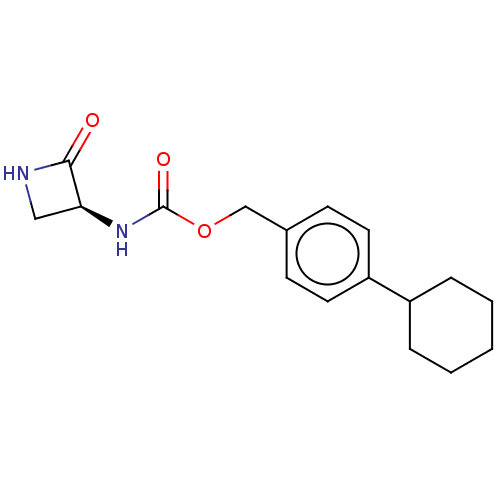
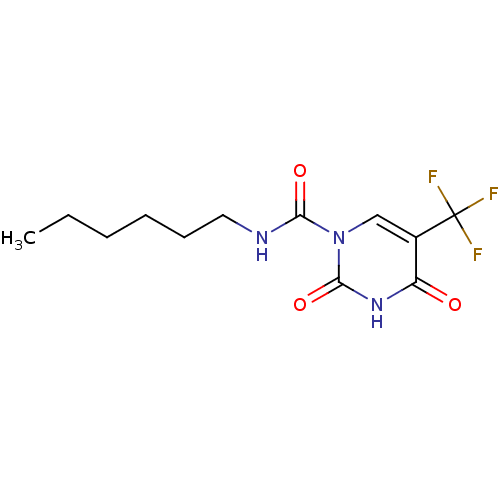
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: 1.48E+4nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: 1.53E+4nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: 1.92E+4nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: 3.72E+4nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetPteridine reductase 1(Leishmania major)
University Of Modena And Reggio Emilia
Curated by ChEMBL
University Of Modena And Reggio Emilia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to...More data for this Ligand-Target Pair
TargetAcid ceramidase(Homo sapiens (Human))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 5.00E+5nMAssay Description:Inhibition of acid ceramidase (unknown origin)More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 4nMpH: 4.5Assay Description:The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub...More data for this Ligand-Target Pair
TargetAcid ceramidase(Rattus norvegicus (Rat))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 5nMpH: 4.5 T: 2°CAssay Description:NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 5nMAssay Description:Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 5nMAssay Description:Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 5nMpH: 4.5 T: 2°CAssay Description:NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 6nMpH: 4.5Assay Description:The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 6.60nMAssay Description:Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS methodMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysisMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMpH: 7.4Assay Description:Lysosomal NAAA protein preparation were obtained by homogenizing male Sprague-Dawley rat lungs (Charles River) in 20 mM Tris-HCl buffer pH 7.4 contai...More data for this Ligand-Target Pair
TargetAcid ceramidase(Rattus norvegicus (Rat))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of Sprague Dawley rat lung native NAAA enzyme using heptadecenoylethanolamide as substrate preincubated for 30 mins followed by substrate ...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 7nMAssay Description:Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 7nMAssay Description:Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 7nMpH: 4.5 T: 2°CAssay Description:NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMpH: 7.4Assay Description:Lysosomal NAAA protein preparation were obtained by homogenizing male Sprague-Dawley rat lungs (Charles River) in 20 mM Tris-HCl buffer pH 7.4 contai...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS methodMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS methodMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 7nMpH: 4.5 T: 2°CAssay Description:NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi...More data for this Ligand-Target Pair
TargetAcid ceramidase(Rattus norvegicus (Rat))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 7.20nMAssay Description:Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS methodMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 7.30nMAssay Description:Inhibition of human recombinant His6-tagged NAAA expressed in HEK293 cells using 10-cis-heptadecenoylethanolamide as substrate after 30 mins by UPLC/...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 8nMpH: 4.5Assay Description:The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 9nMpH: 4.5Assay Description:The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 9nMpH: 4.5 T: 2°CAssay Description:NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 10nMpH: 4.5 T: 2°CAssay Description:NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS methodMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS methodMore data for this Ligand-Target Pair
TargetAcid ceramidase(Rattus norvegicus (Rat))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysisMore data for this Ligand-Target Pair
TargetAcid ceramidase(Rattus norvegicus (Rat))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
University of California
US Patent
University of California
US Patent
Affinity DataIC50: 12nMpH: 4.5Assay Description:The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub...More data for this Ligand-Target Pair