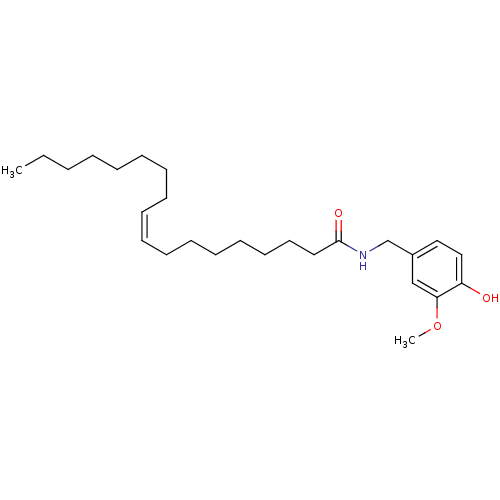
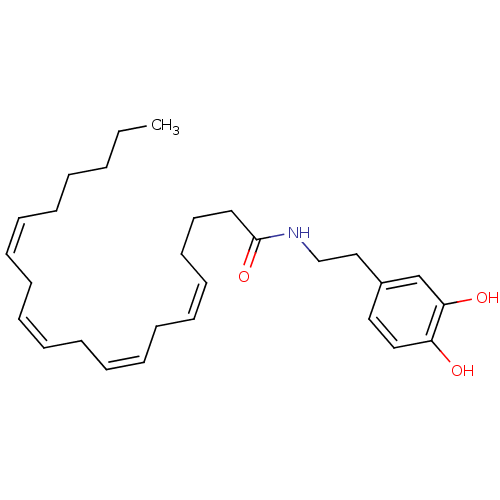
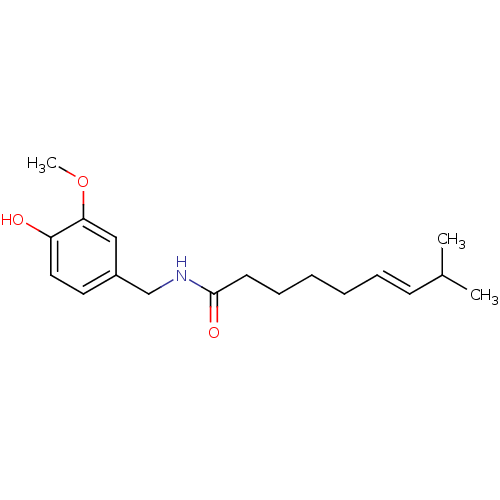
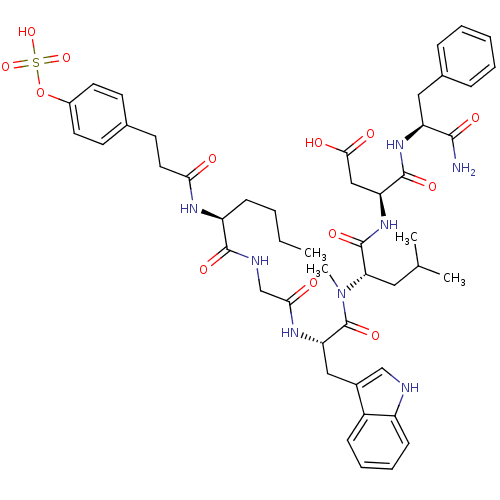
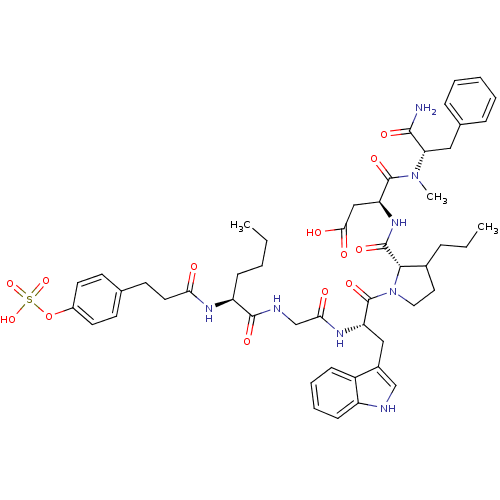
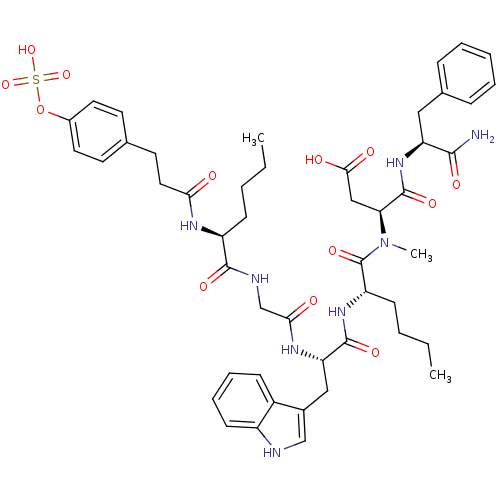
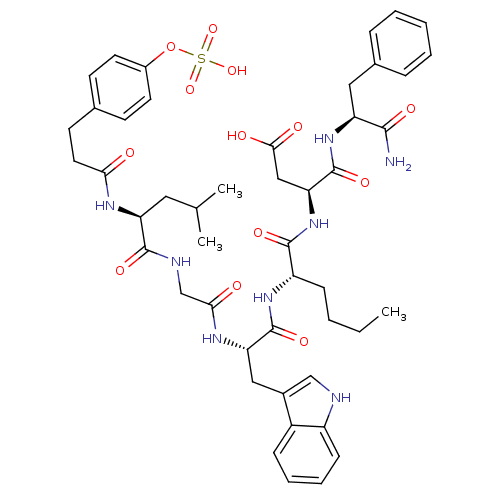
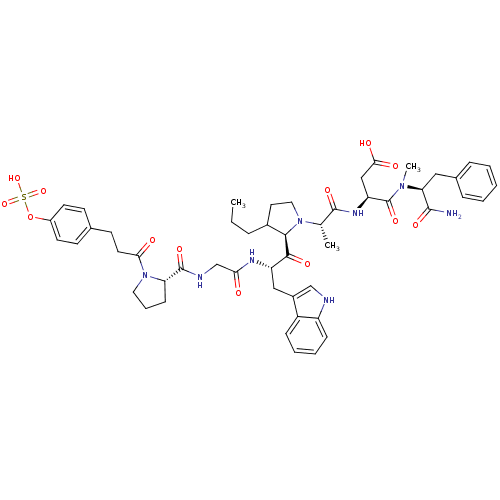
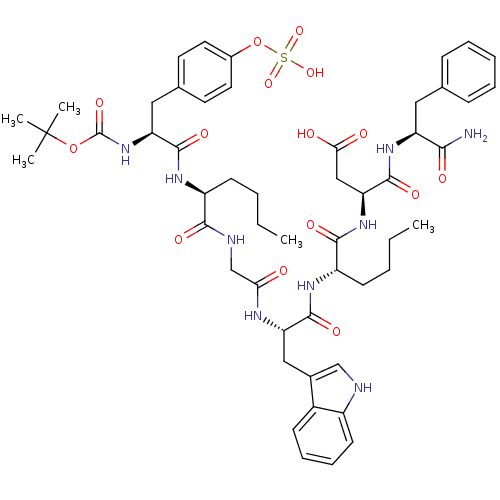
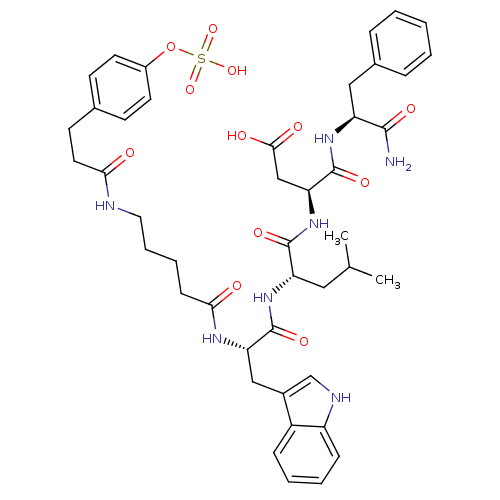
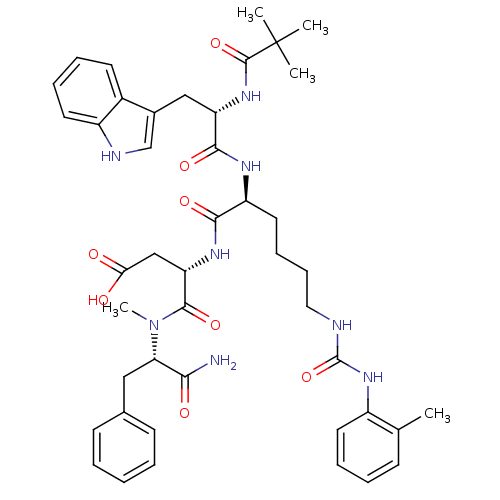
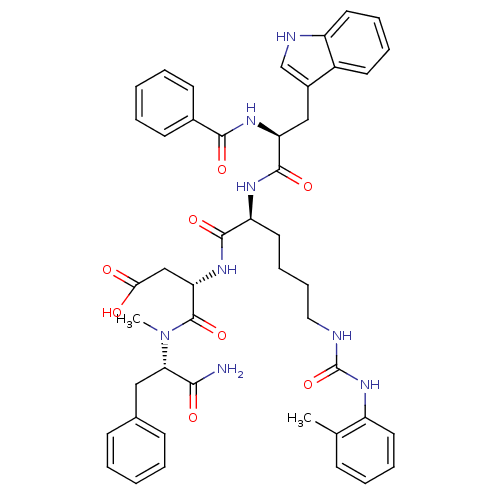
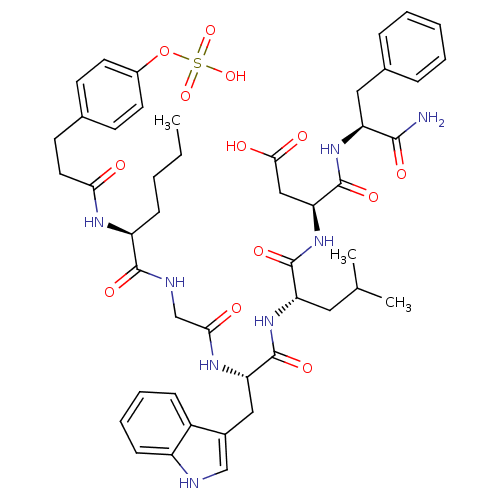
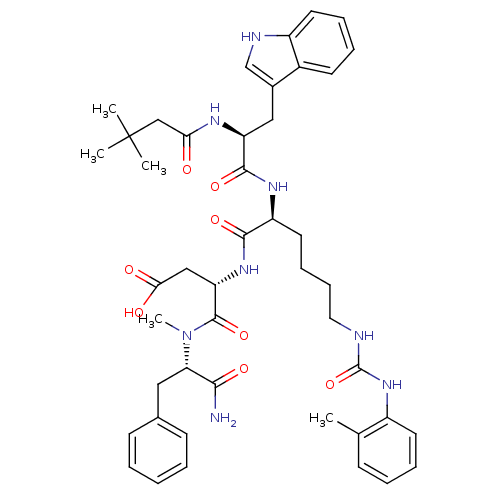
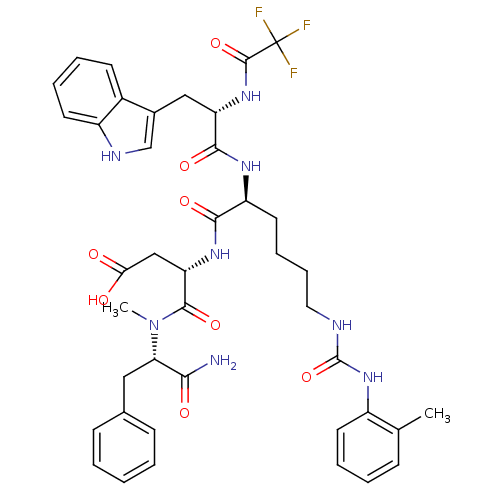
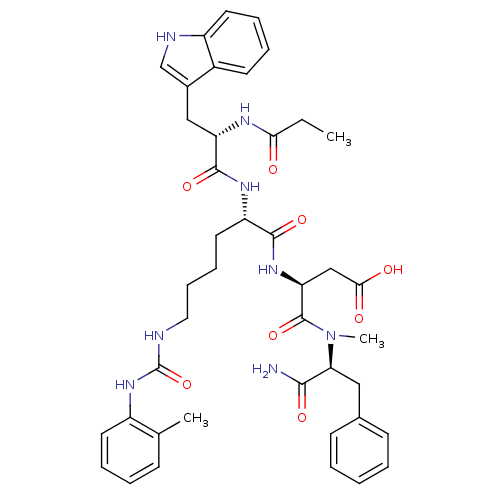
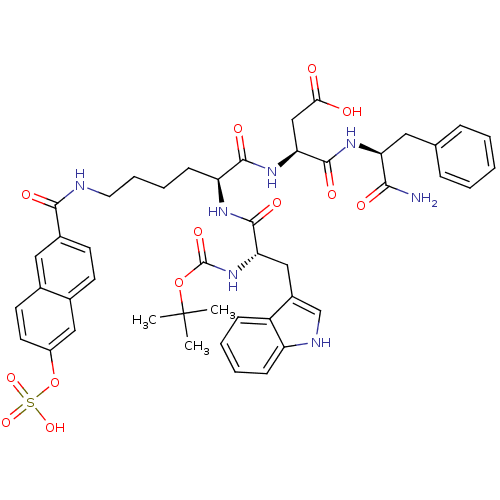
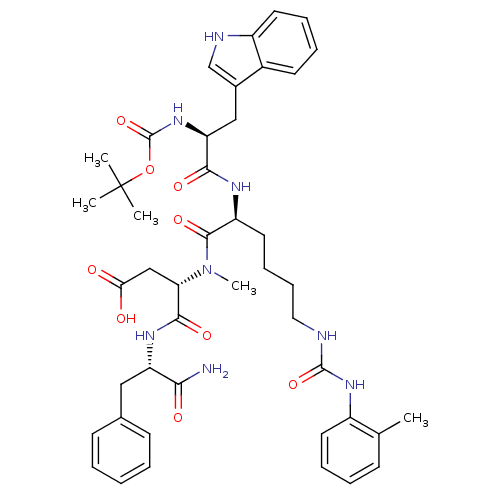
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
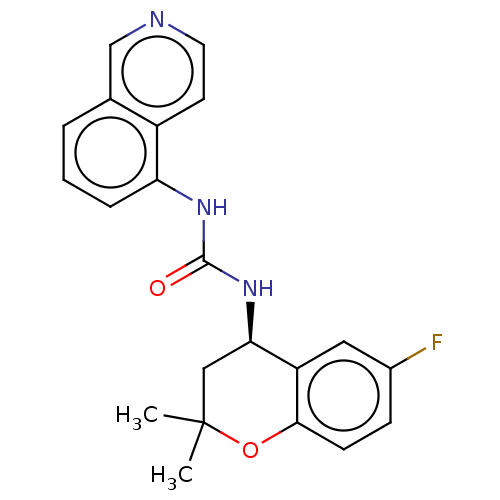
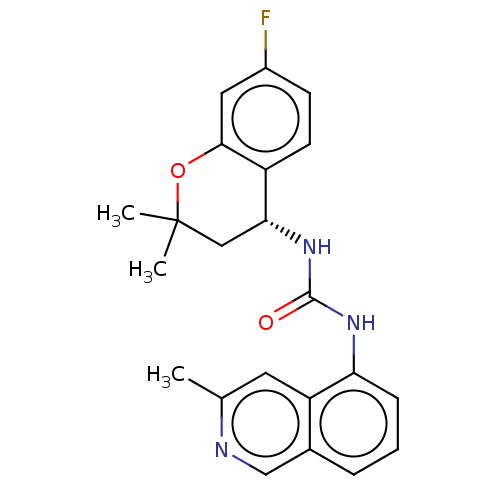
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 7nM ΔG°: -46.5kJ/mole EC50: 5nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 27nM ΔG°: -43.2kJ/mole EC50: 34nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 47nM ΔG°: -41.8kJ/mole EC50: 11nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 58nM ΔG°: -41.3kJ/mole EC50: 46nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 65nM ΔG°: -41.0kJ/mole EC50: 24nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 71nM ΔG°: -40.8kJ/mole EC50: 74nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 112nM ΔG°: -39.7kJ/mole EC50: 34nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 589nM ΔG°: -35.6kJ/mole EC50: 129nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 603nM ΔG°: -35.5kJ/mole EC50: 95nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 1.29E+3nM ΔG°: -33.6kJ/mole EC50: 282nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 1.59E+3nM ΔG°: -33.1kJ/mole EC50: 132nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(GUINEA PIG)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: >6.31E+3nM ΔG°: >-29.7kJ/mole EC50: 1.48E+3nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 2.00E+4nM ΔG°: -26.8kJ/mole EC50: 29nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
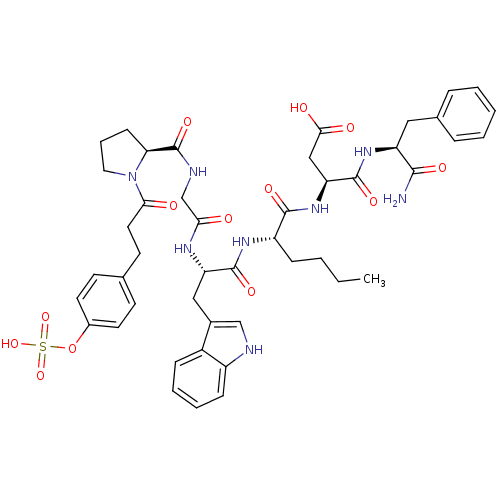
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
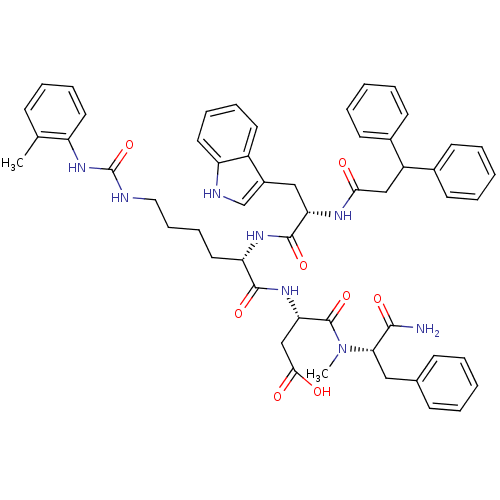
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
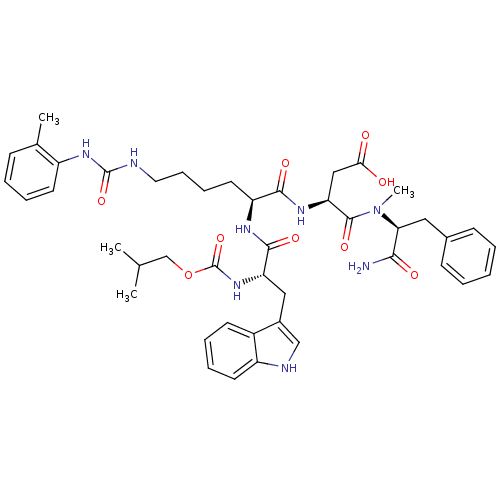
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreasMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.590nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreasMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 0.930nMAssay Description:In vitro ability to inhibit [3H]propionyl-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 0.990nMAssay Description:In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 0.990nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreasMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of specific binding of [125I]BH-CCK-8 in guinea pig pancreas.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreasMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair

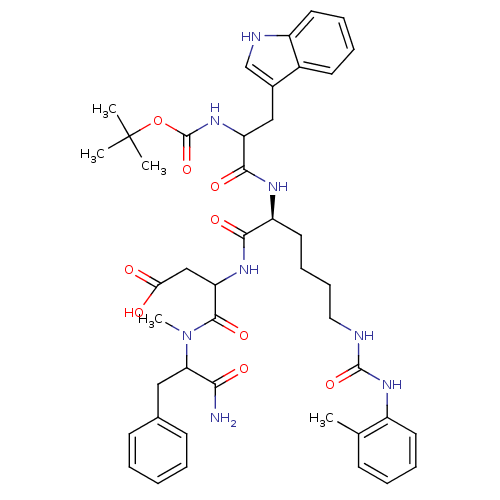

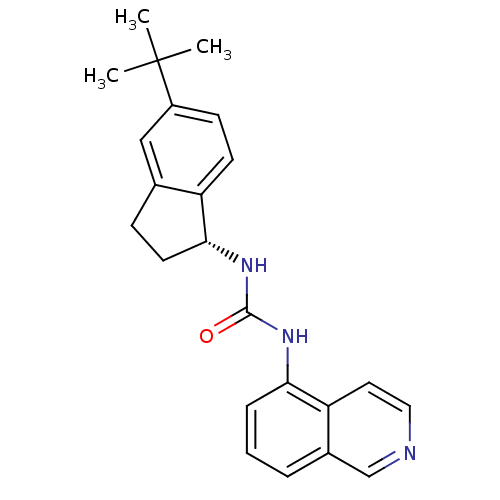
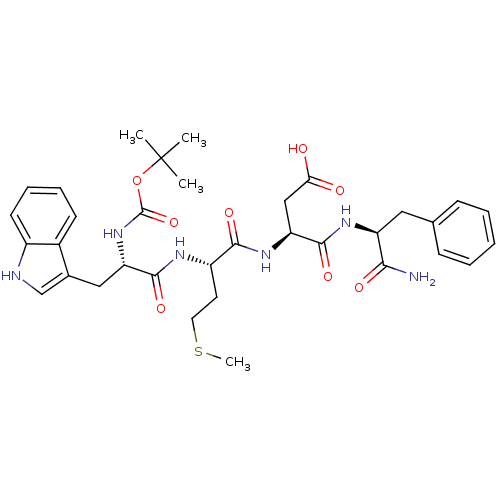
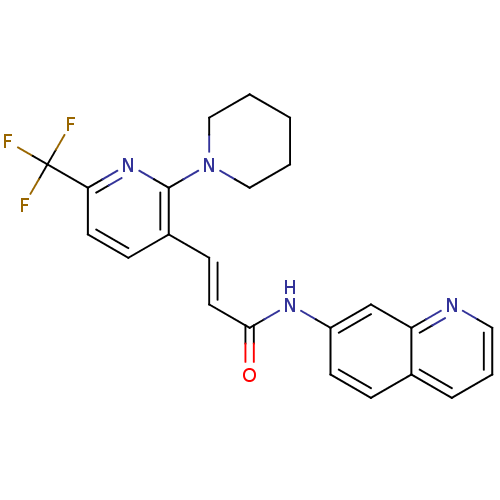
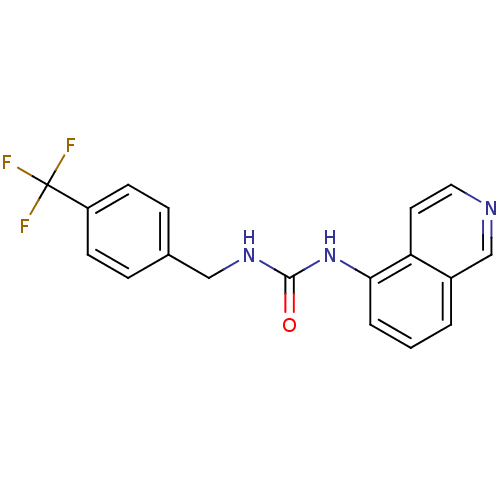

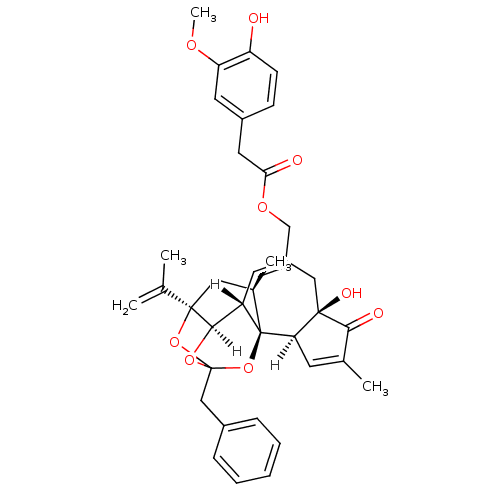
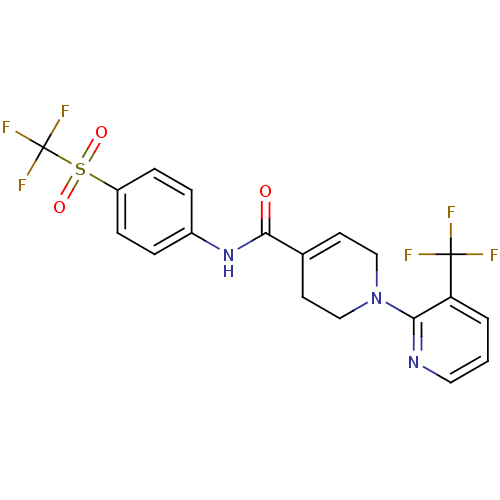

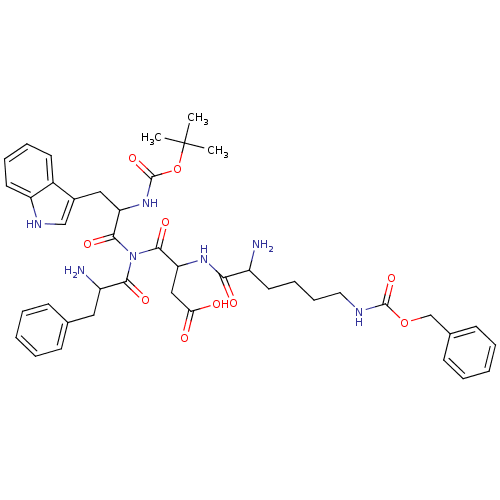

 3D Structure (crystal)
3D Structure (crystal)