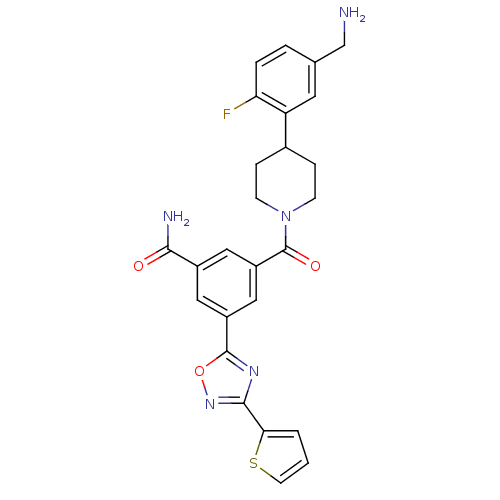
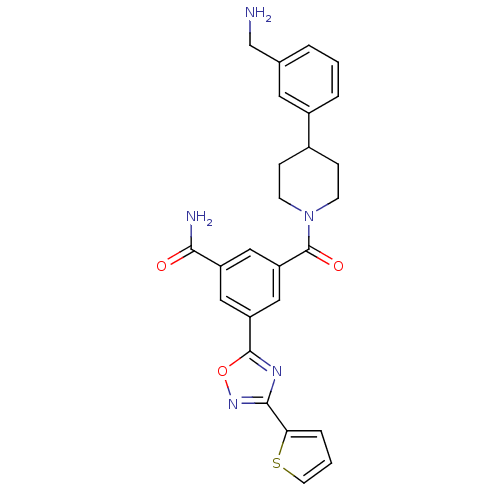
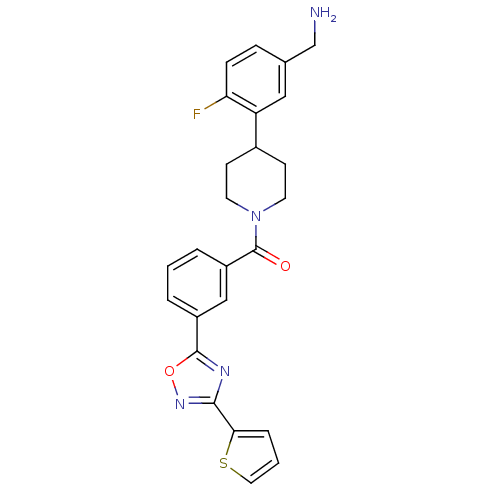
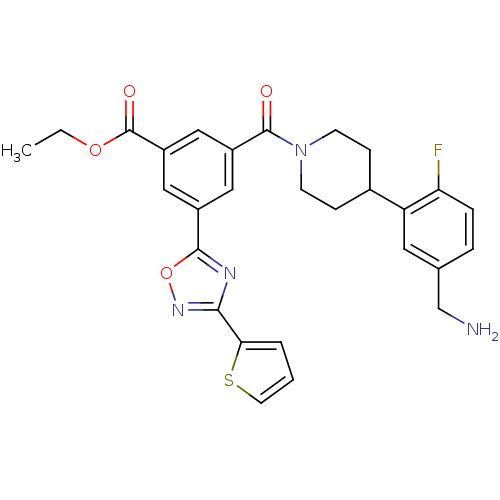
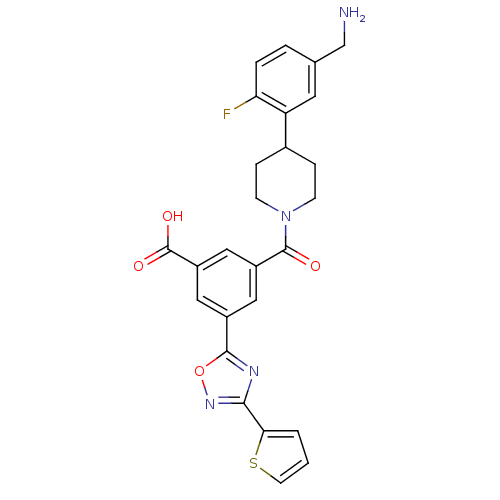
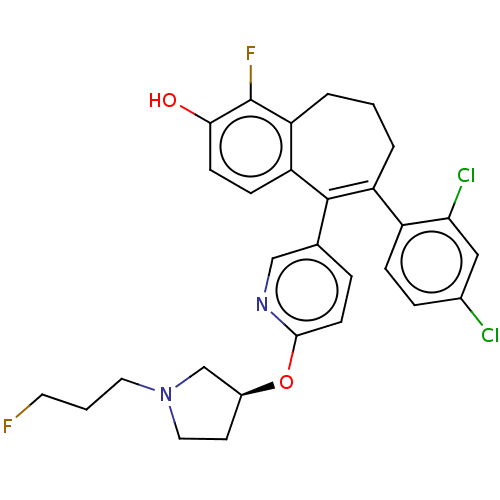
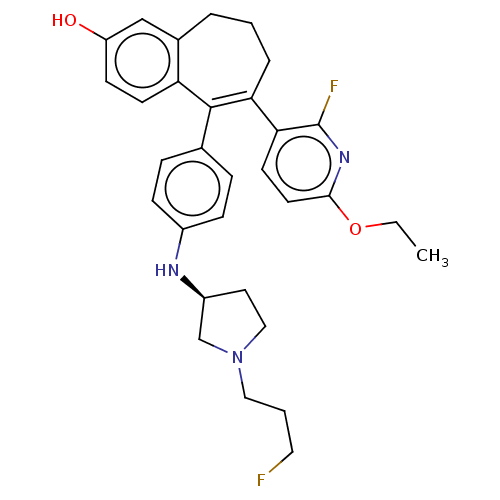
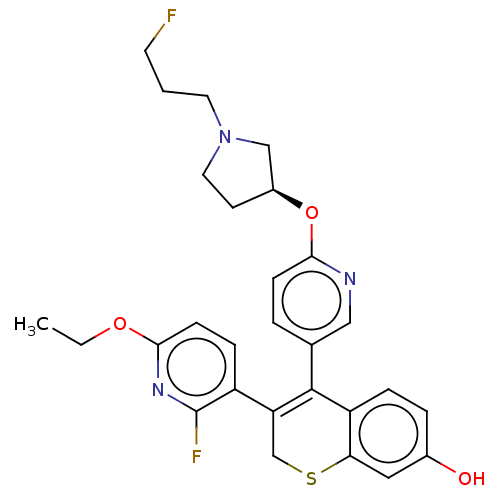
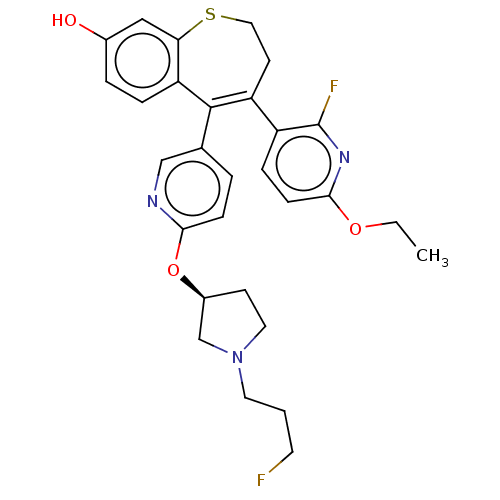
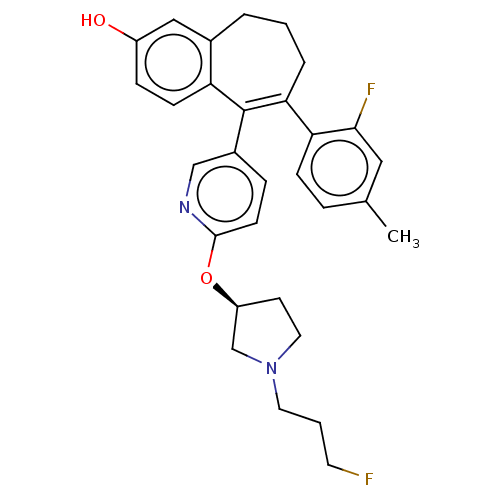
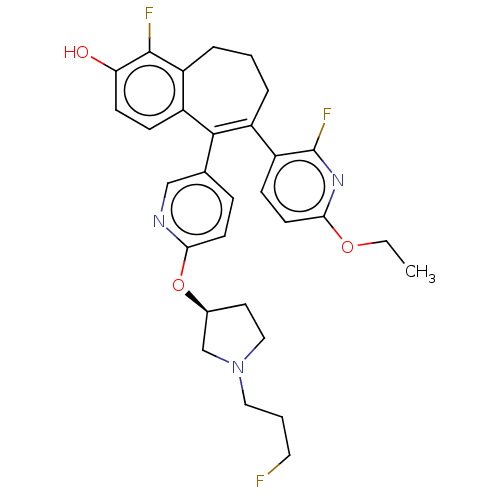
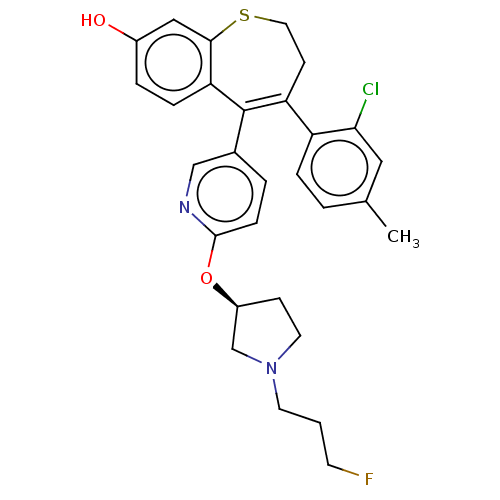
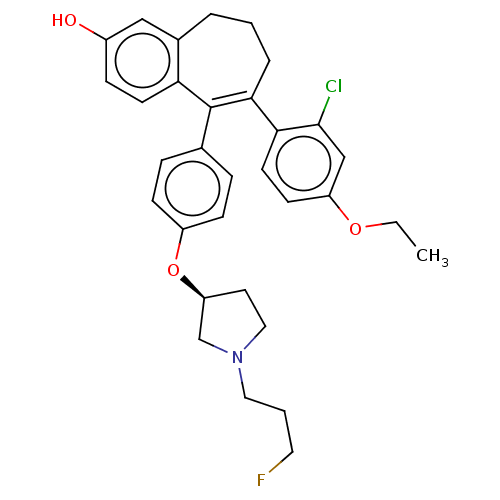
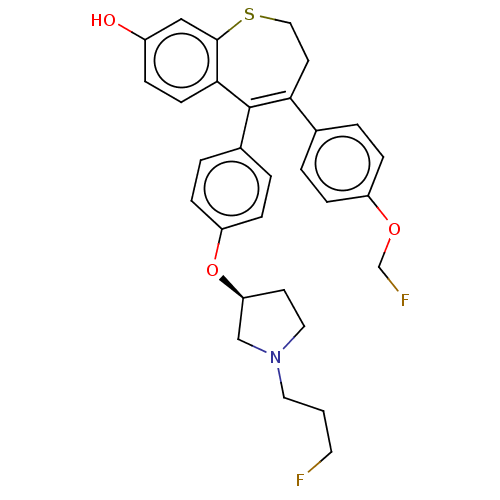
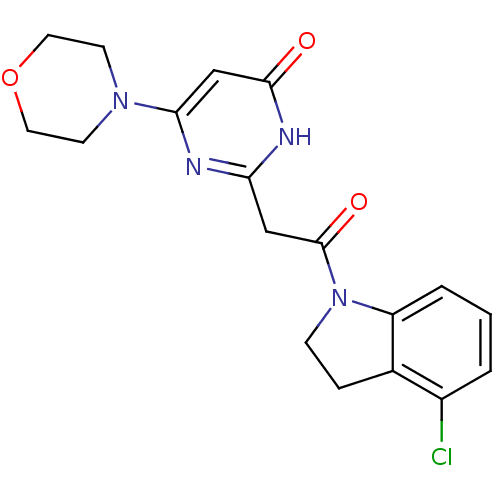
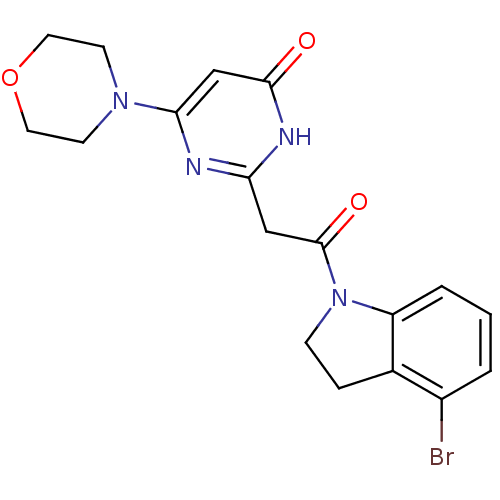
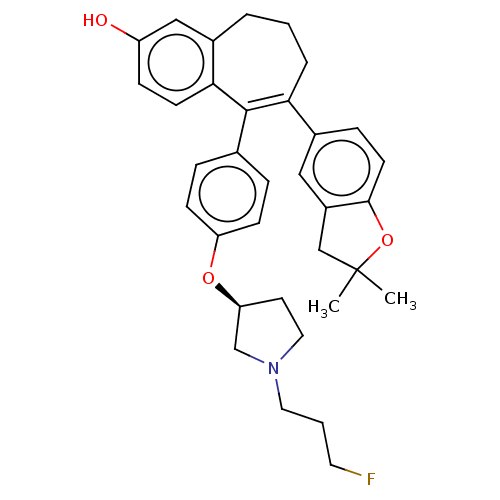
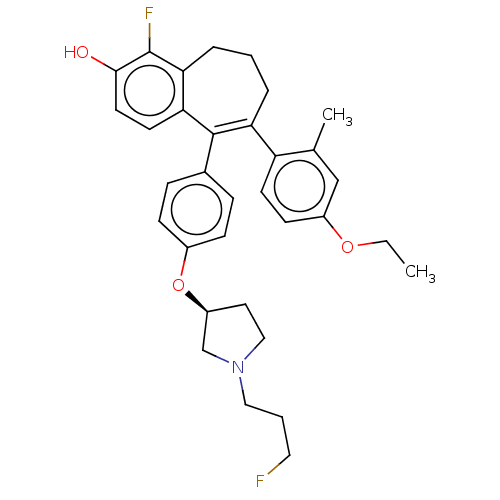
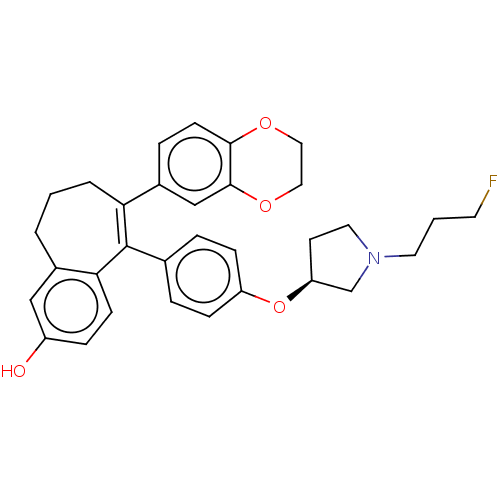
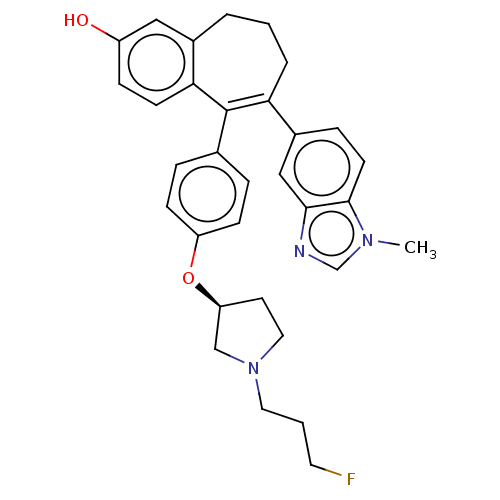
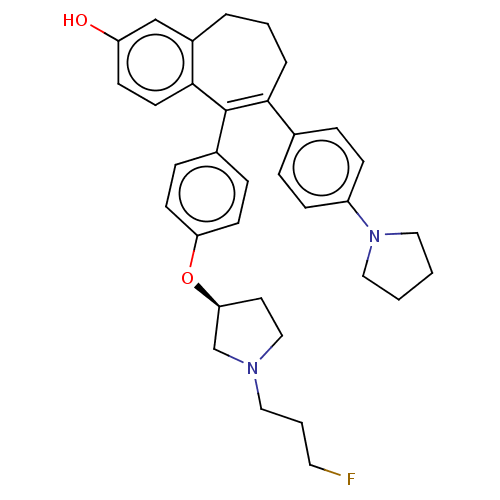
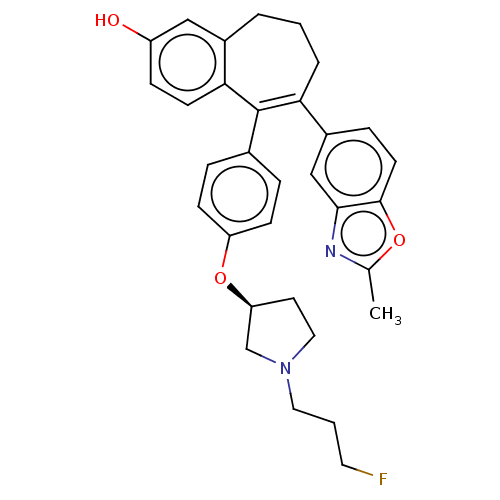
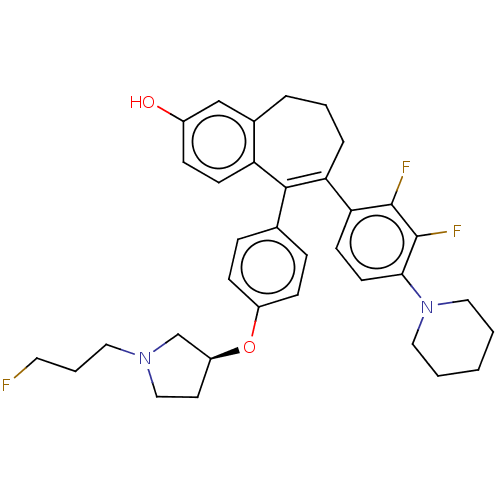
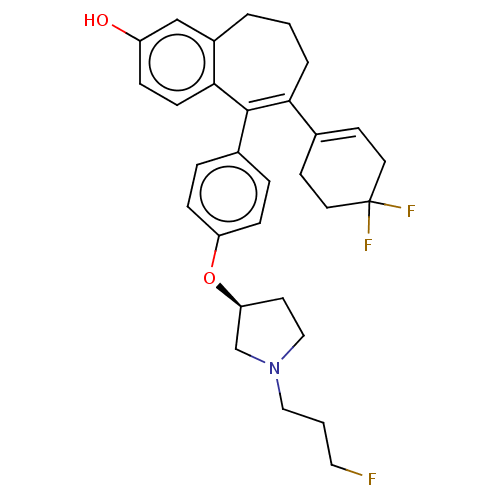
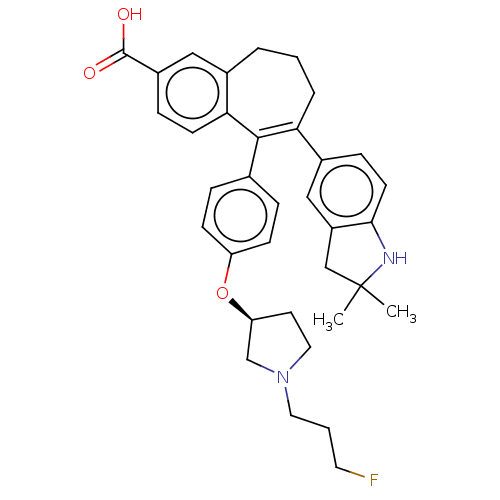
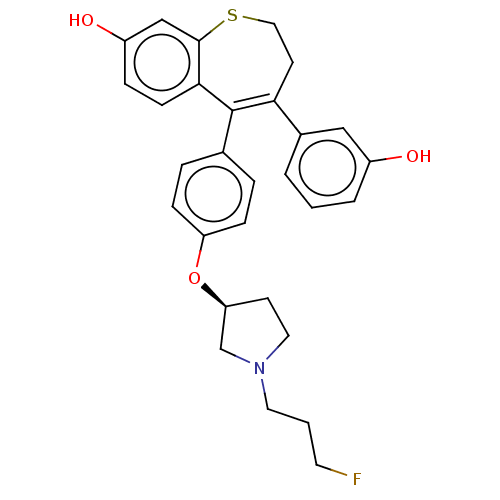
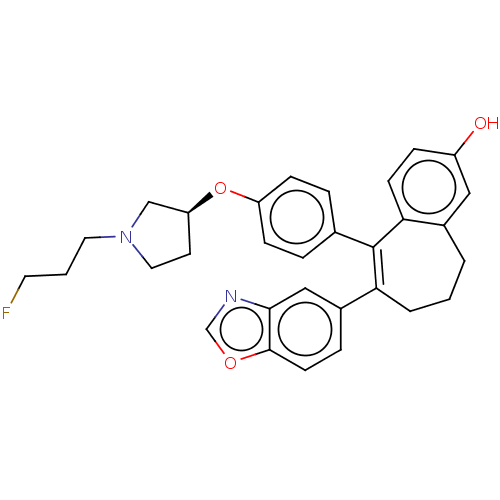
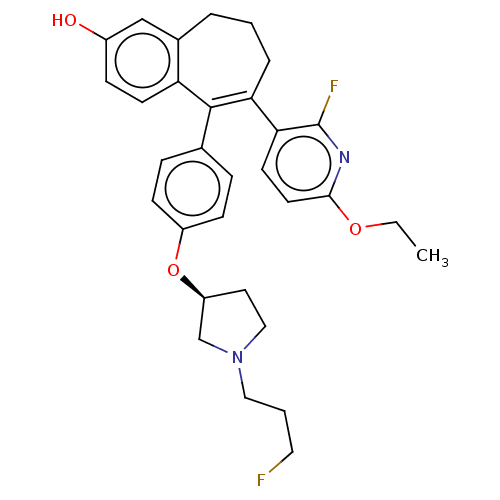
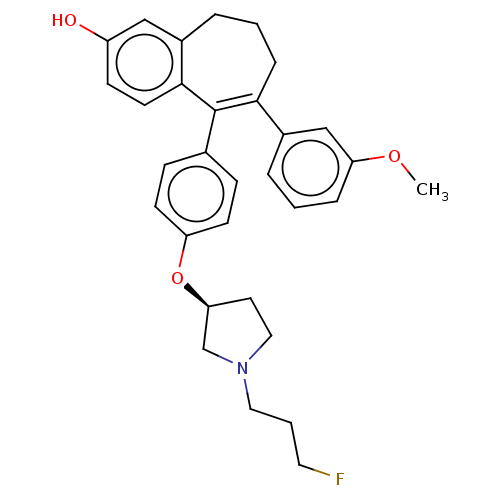
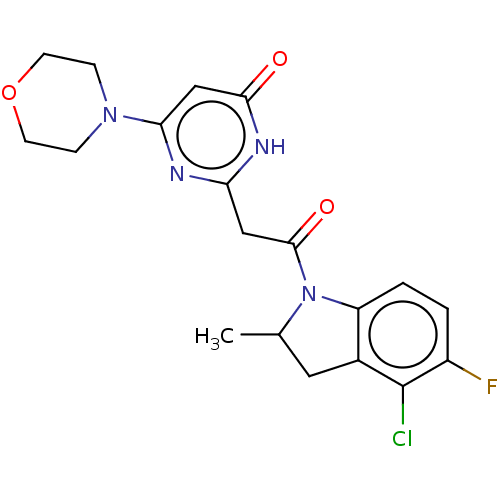
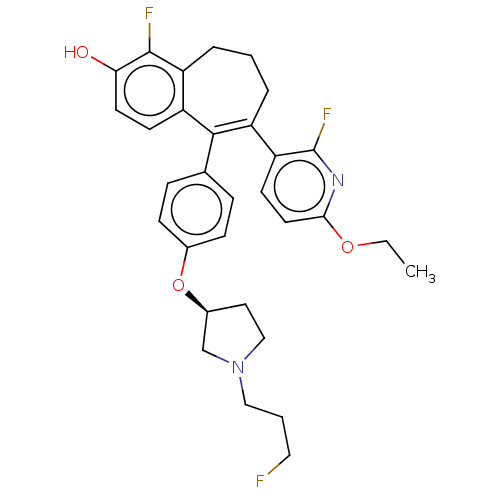
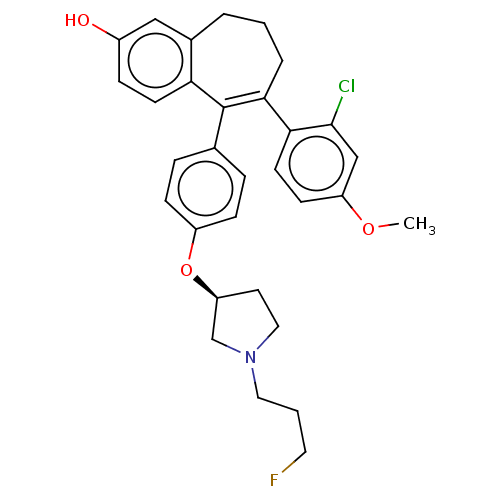
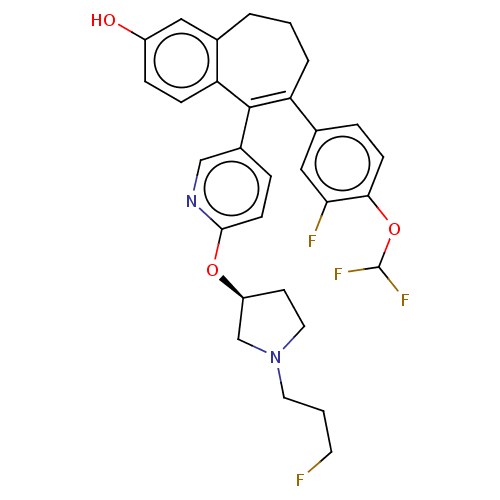
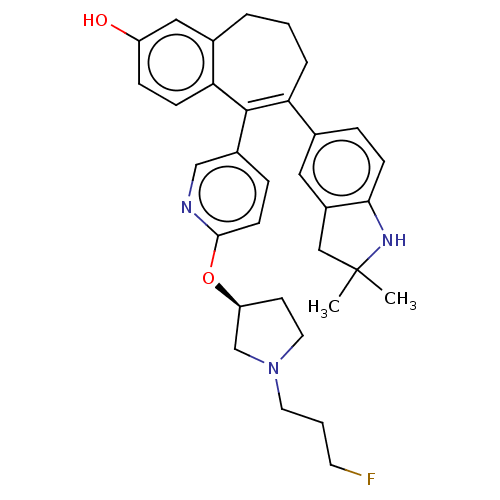
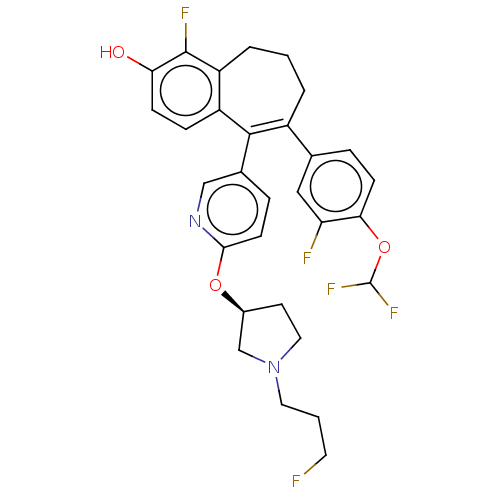
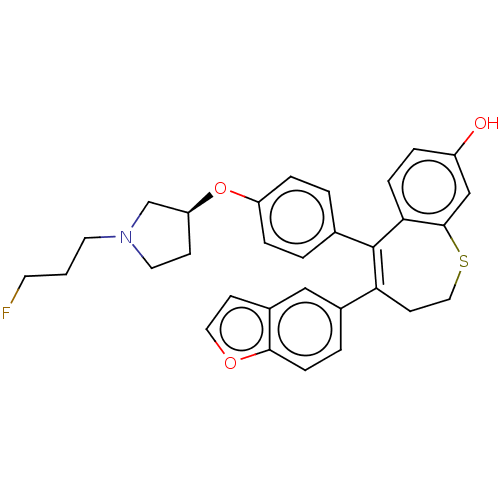
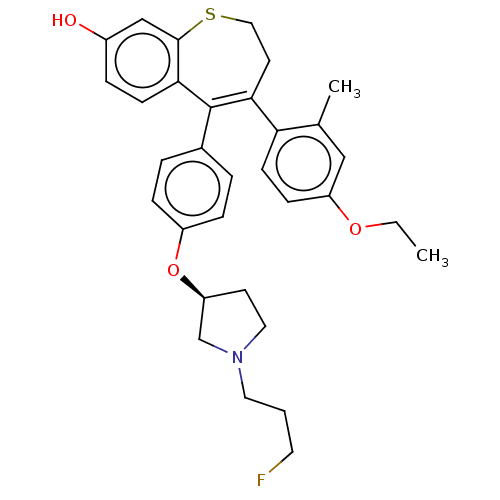
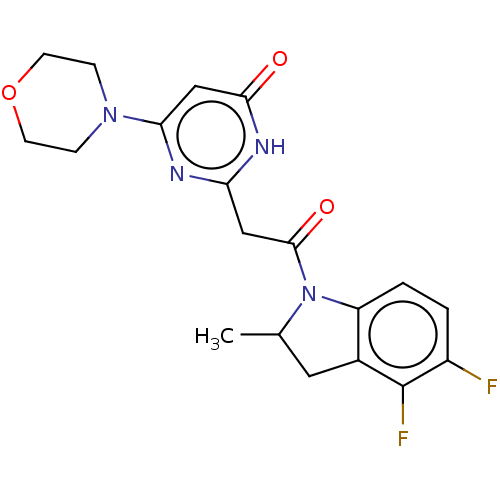
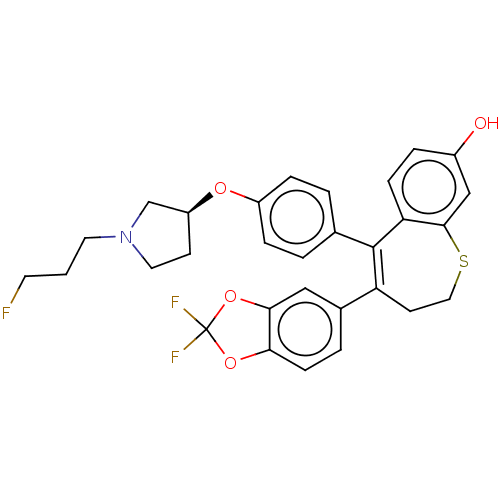
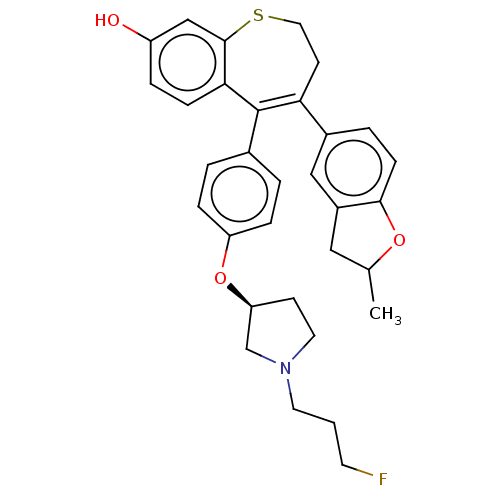
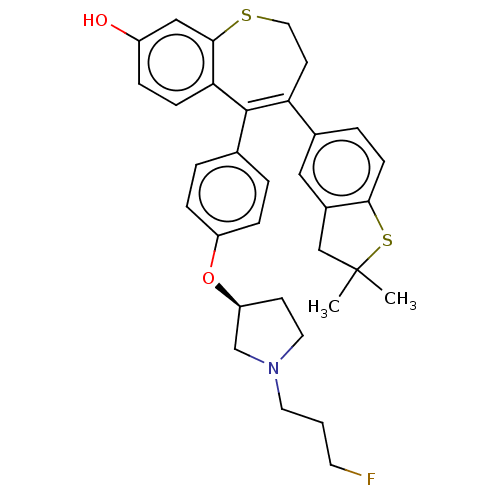
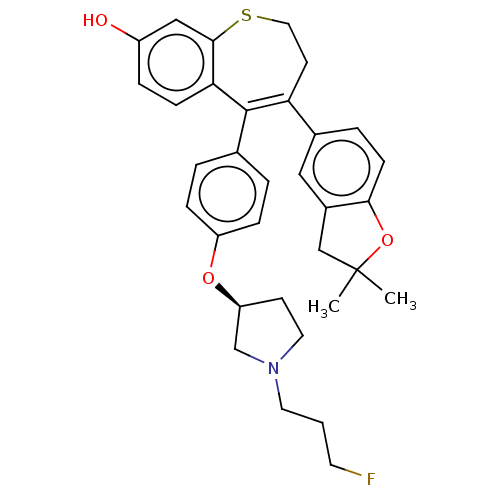
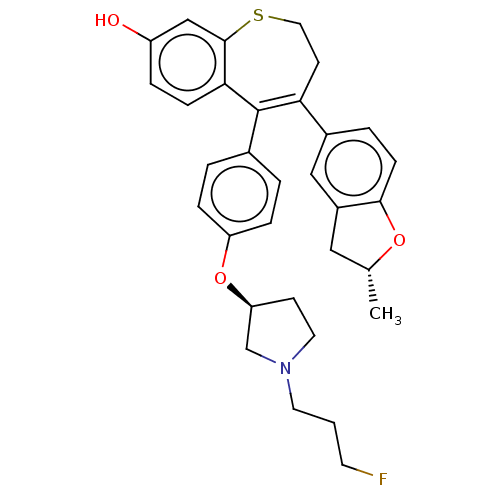
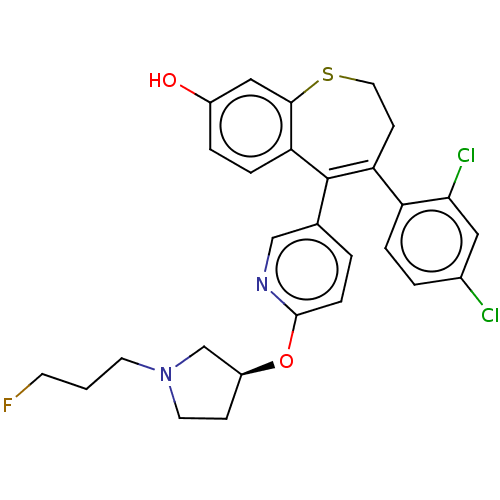
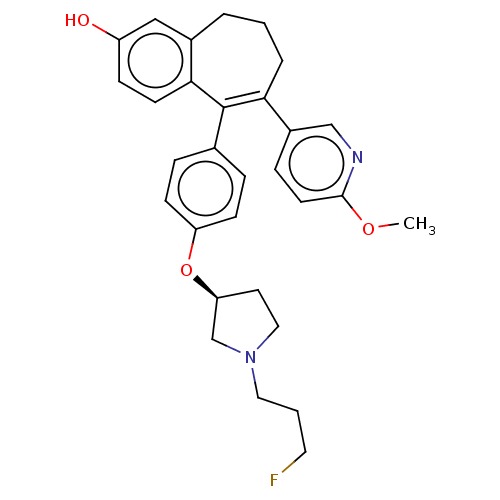
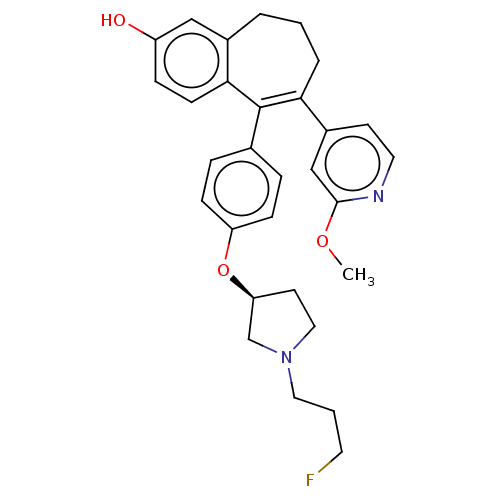
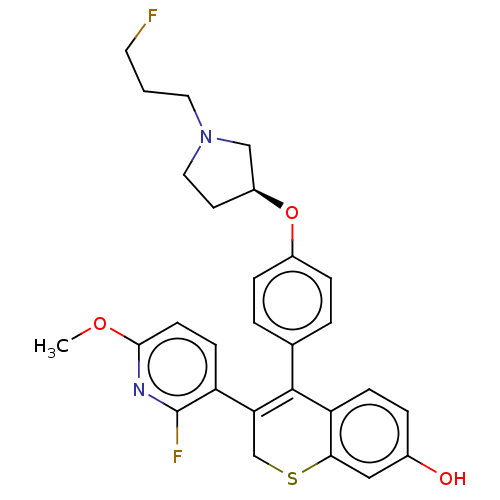
Affinity DataKi: 1.30nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataKi: 88nMAssay Description:Inhibitory activity against human mast cell tryptase betaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:This test is based on measurement of the expression of AKT protein phosphorylated on serine 473 (P-AKT-S473) in the PC3 human prostate carcinoma line...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:This test is based on measurement of the expression of AKT protein phosphorylated on serine 473 (P-AKT-S473) in the PC3 human prostate carcinoma line...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Sanofi
Curated by ChEMBL
Sanofi
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Sanofi
Curated by ChEMBL
Sanofi
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:This test is based on measurement of the expression of AKT protein phosphorylated on serine 473 (P-AKT-S473) in the PC3 human prostate carcinoma line...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:This test is based on measurement of the expression of AKT protein phosphorylated on serine 473 (P-AKT-S473) in the PC3 human prostate carcinoma line...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com...More data for this Ligand-Target Pair