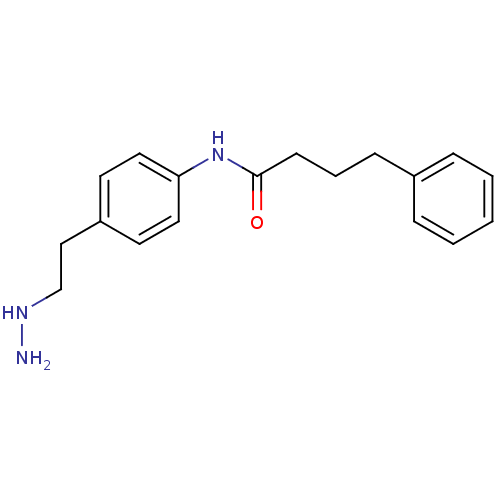
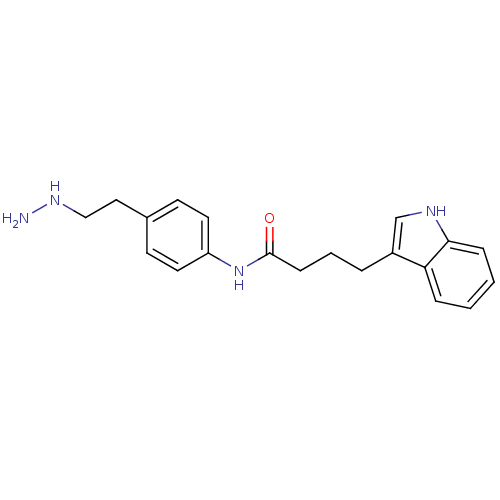
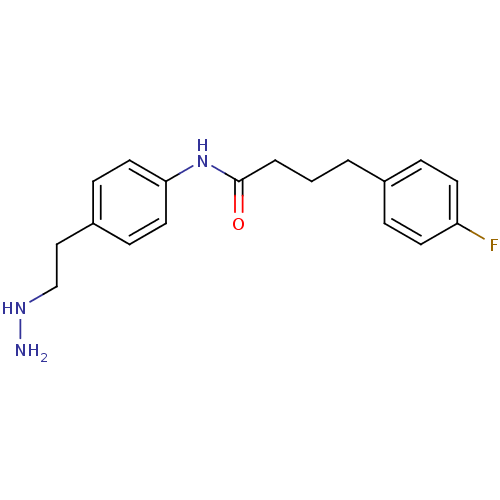
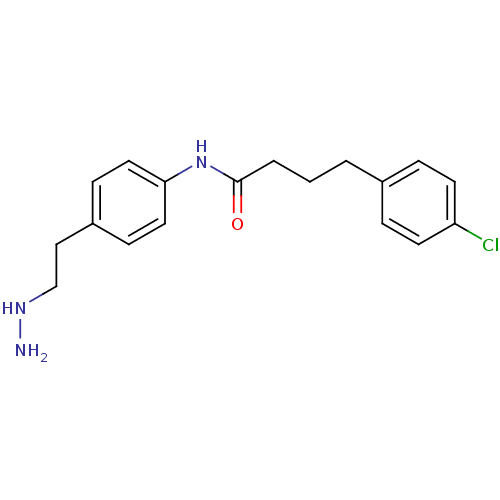
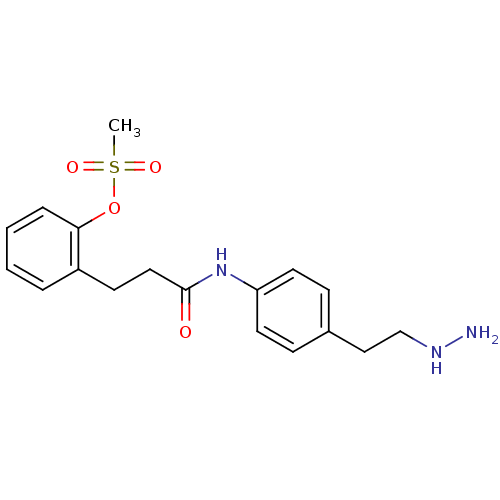
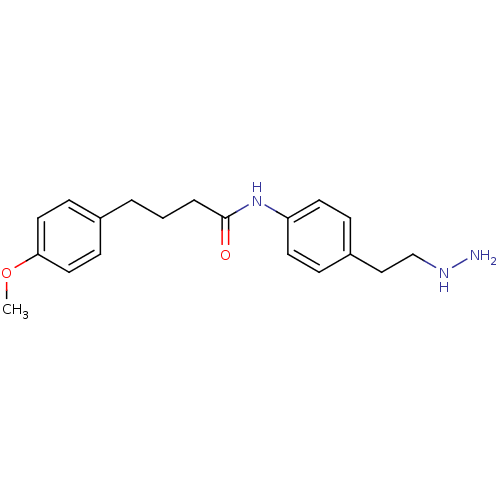
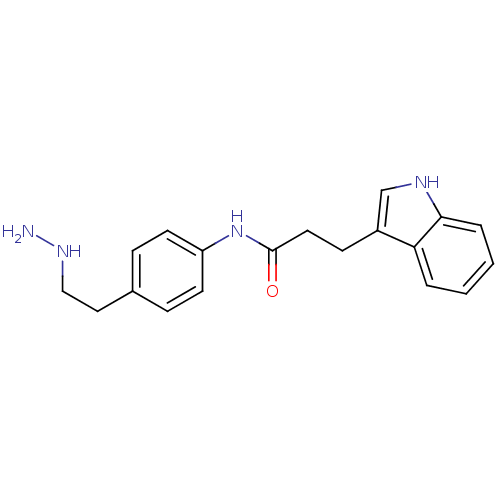
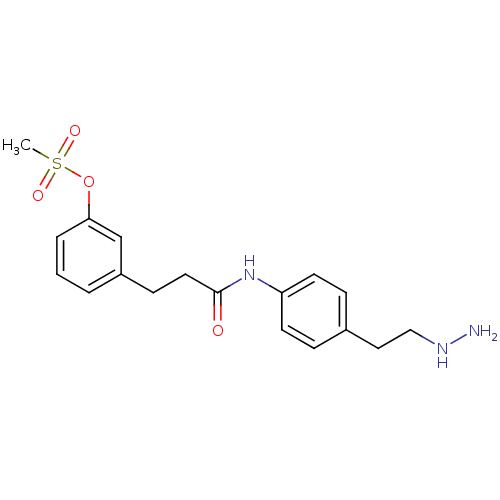
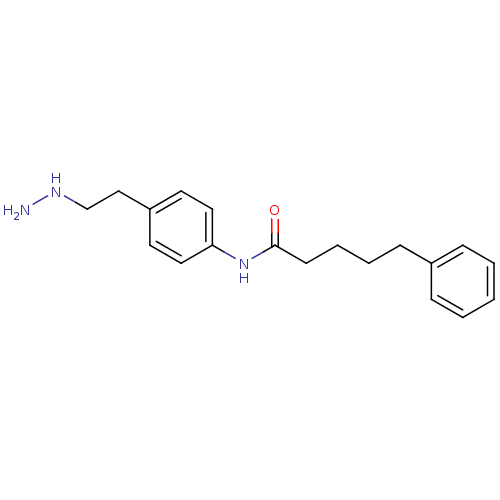
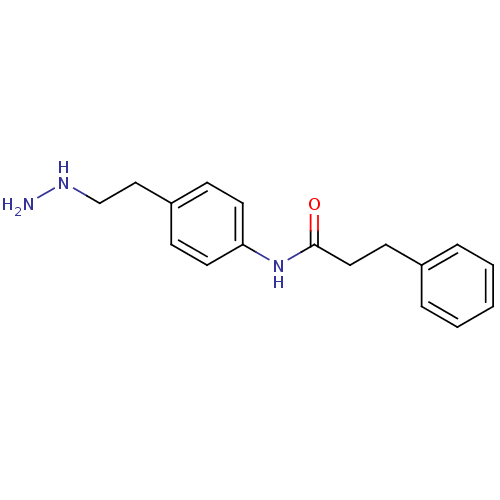
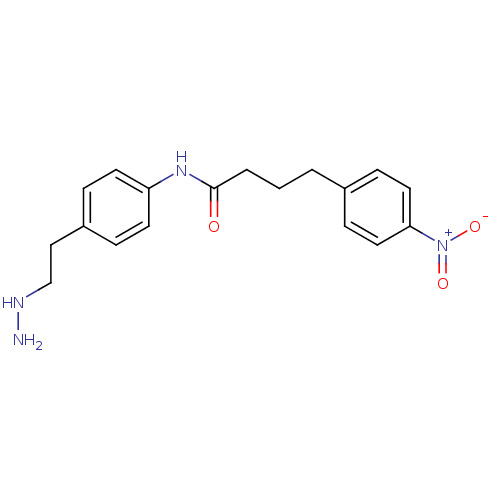
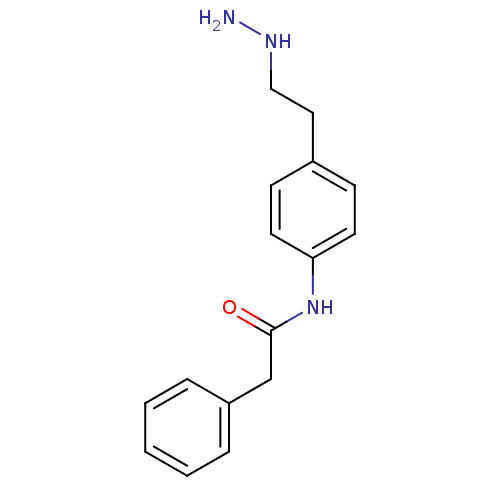
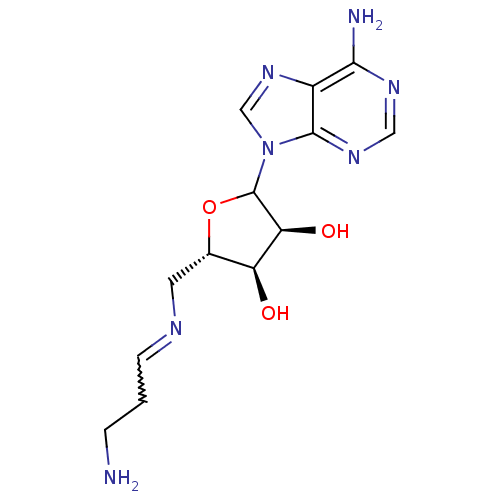
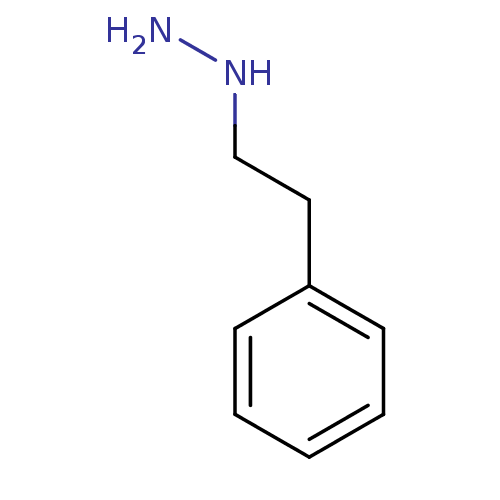
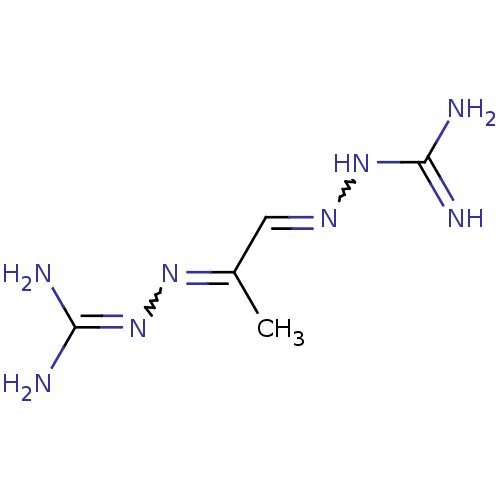
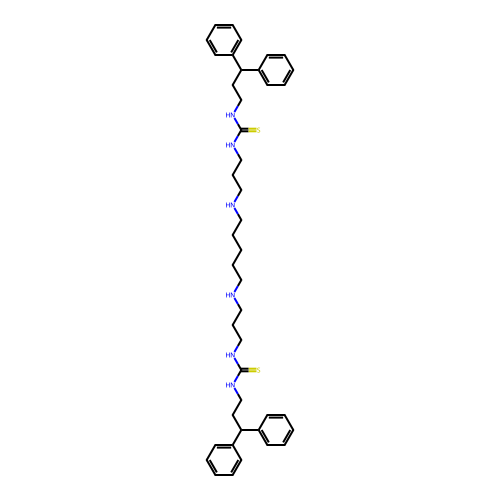
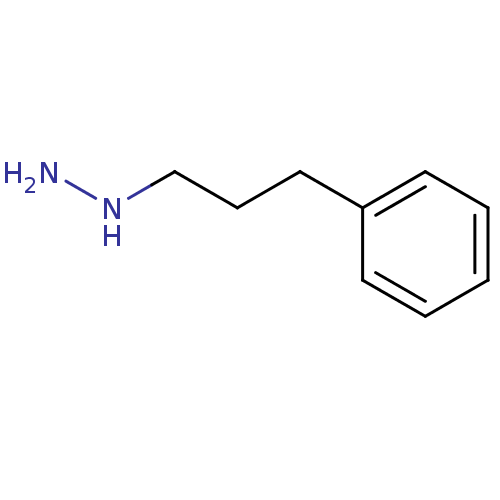
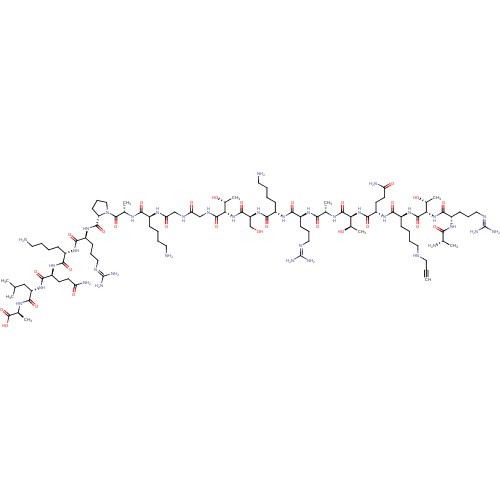
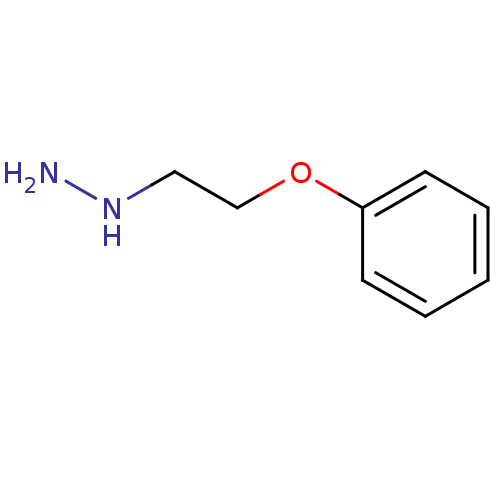
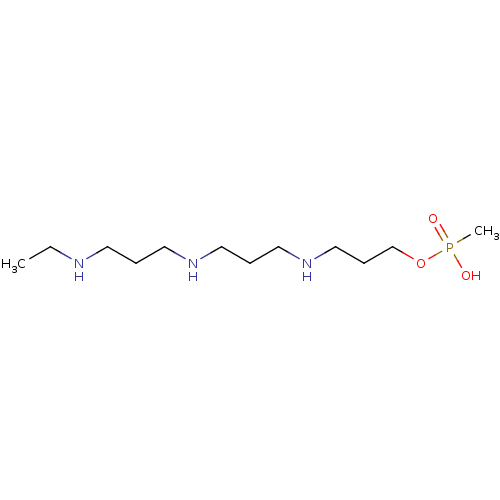
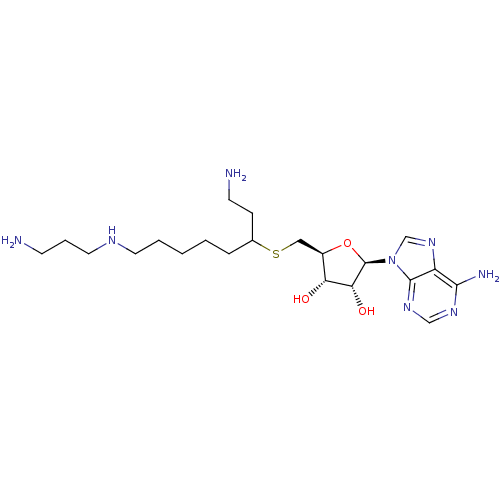
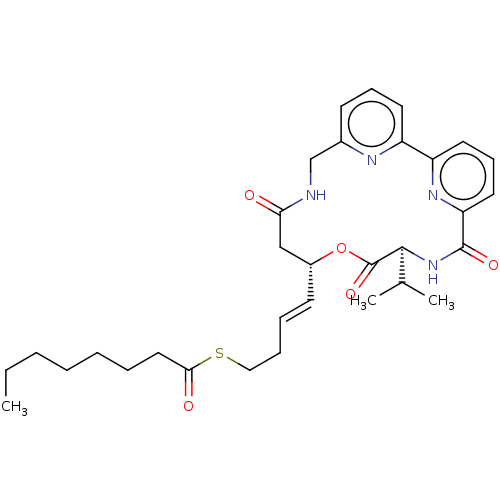
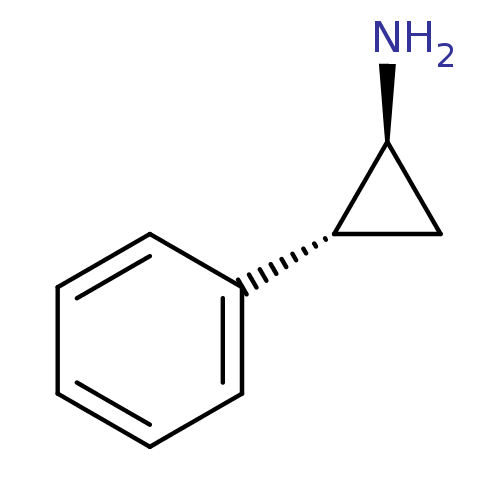
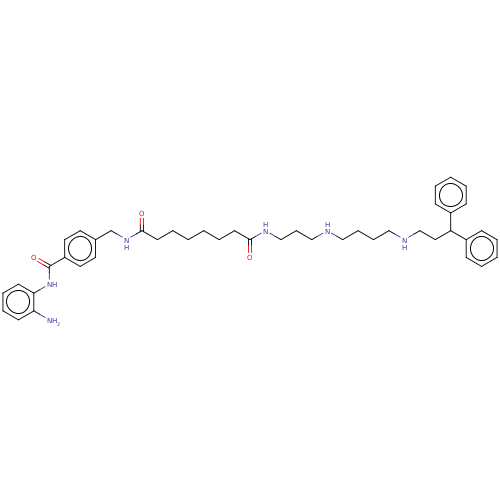
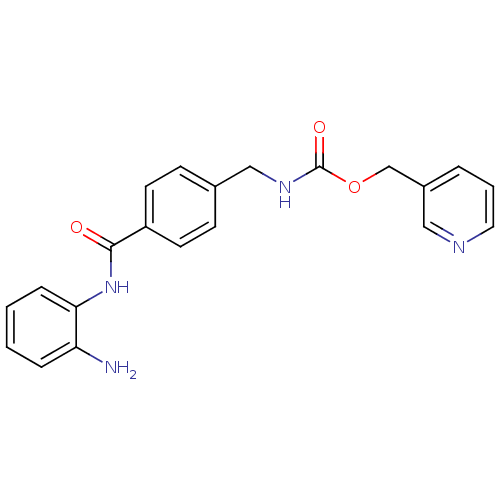
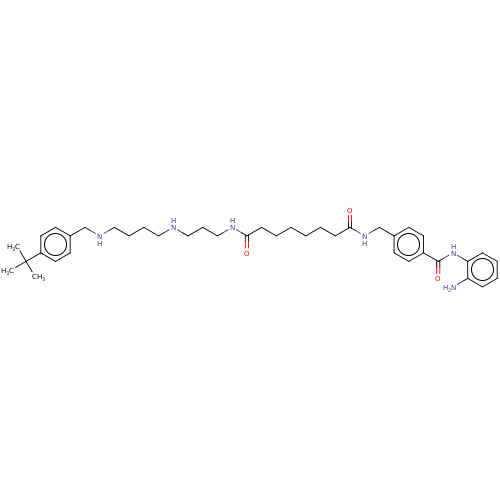
Affinity DataKi: 59nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 100nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 100nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 138nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 156nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 204nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 207nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 210nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 223nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 260nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 260nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 280nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 282nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 370nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Rattus norvegicus)
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 560nMAssay Description:Inhibition of rat liver form of S-adenosyl-methionine decarboxylase enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 820nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO...More data for this Ligand-Target Pair
Affinity DataKi: 900nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of rat liver form of S-adenosyl-methionine decarboxylase enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:Competitive inhibition of human recombinant LSD1 by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO...More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 5.60E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO...More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 1.66E+4nMAssay Description:Time-dependent inhibition of recombinant LSD1 catalytic domain (178 to 831 amino acids) (unknown origin) expressed in baculovirus infected insect Sf9...More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+4nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 4.40E+4nMpH: 7.5Assay Description:Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nMAssay Description:Inhibitory activity against human SSATMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against spermine synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of recombinant HDAC1 (unknown origin) incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substra...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant HDAC3 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of recombinant HDAC2 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant HDAC3 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibitory activity against spermine synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 61nMAssay Description:Inhibition of recombinant HDAC1 (unknown origin) incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substra...More data for this Ligand-Target Pair
Affinity DataIC50: 64nMAssay Description:Inhibition of recombinant HDAC2 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of recombinant HDAC6 (unknown origin)incubated for 15 mins before substrate addition and measured after 30 to 45 mins by HDAC-Glo substrat...More data for this Ligand-Target Pair
Affinity DataIC50: <2.00E+3nMAssay Description:Inhibition of LSD1More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of human HDAC in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of MAO-A (unknown origin) by MAo-Glo kit analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of human recombinant LSD1 after 30 mins to 4 hrs by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of HDAC from human HeLa cells assessed as of histone H3 acetylationMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of HDAC from human HeLa cells assessed as of histone H3 acetylationMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of human HDAC in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of MAO-B (unknown origin) by MAo-Glo kit analysisMore data for this Ligand-Target Pair