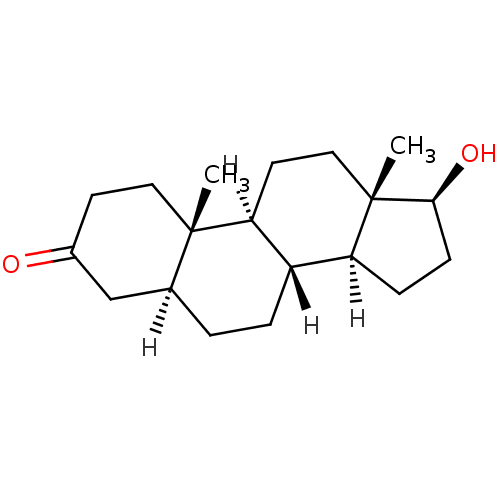
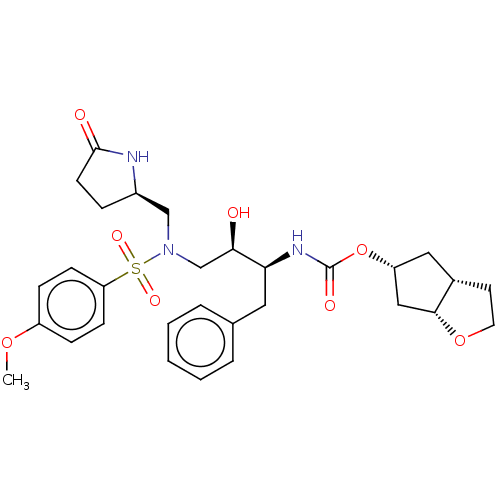
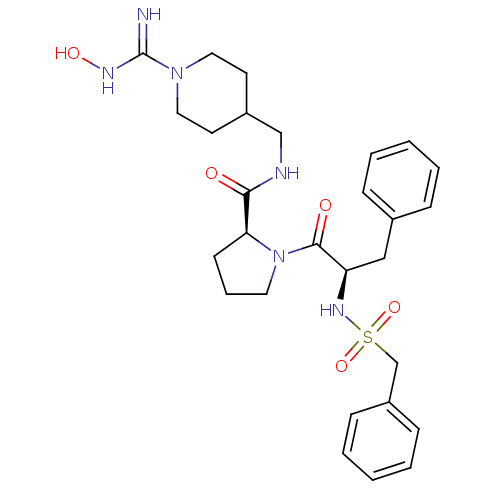
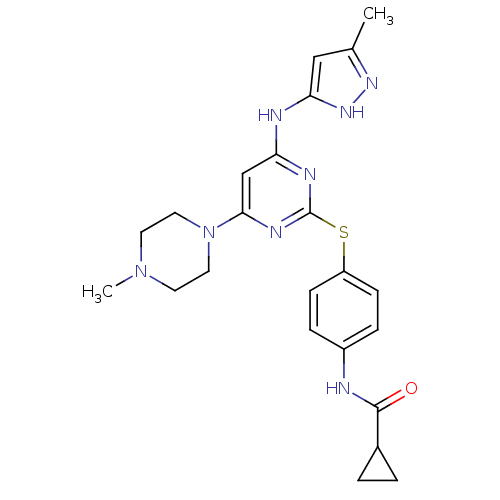
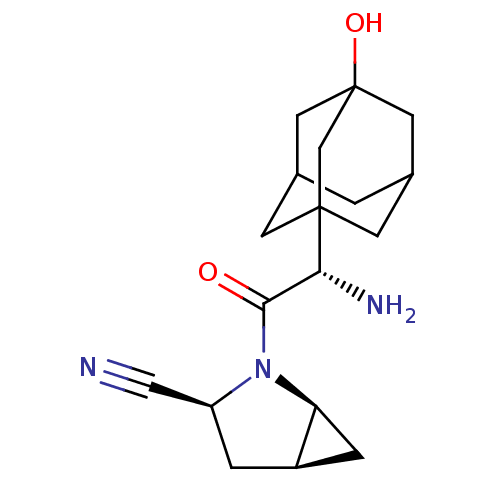
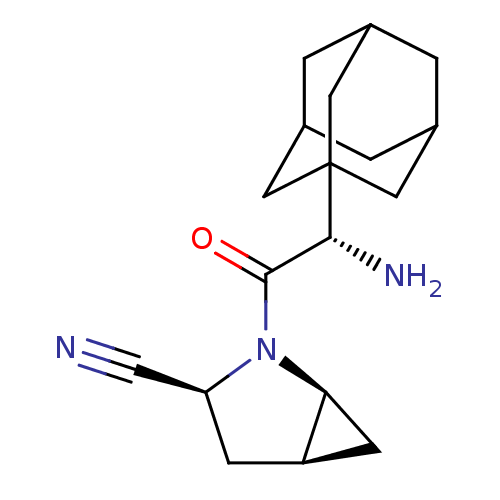
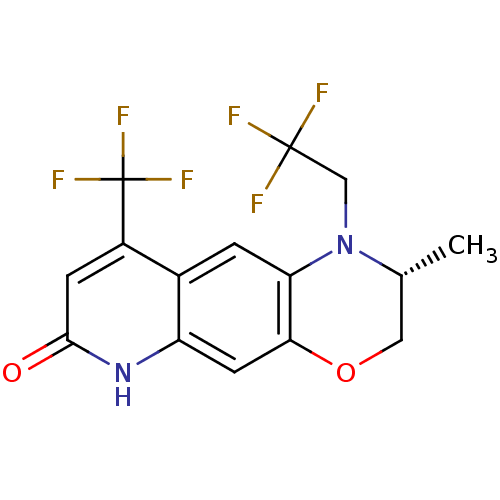
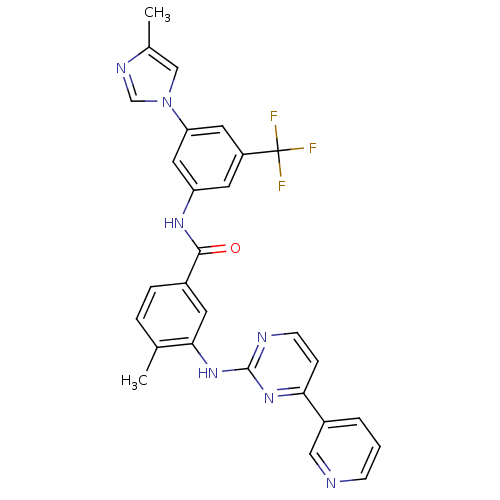
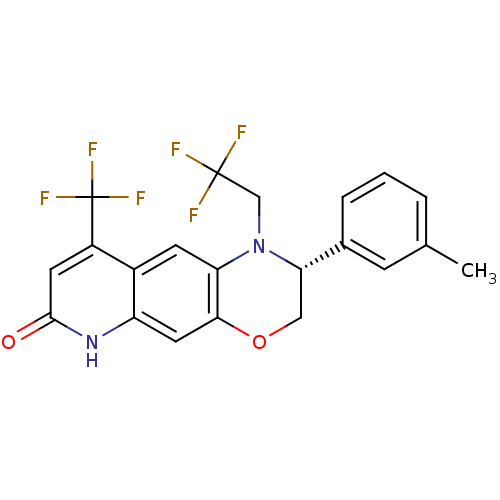
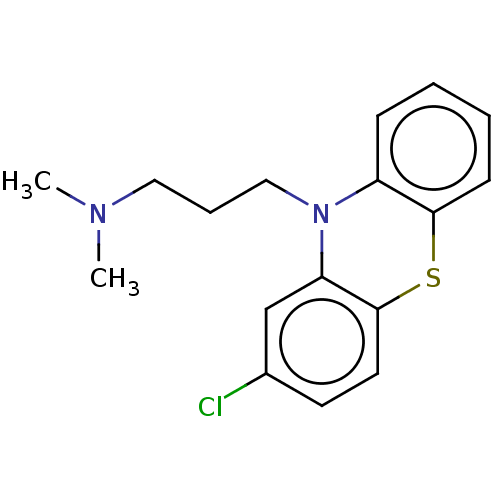
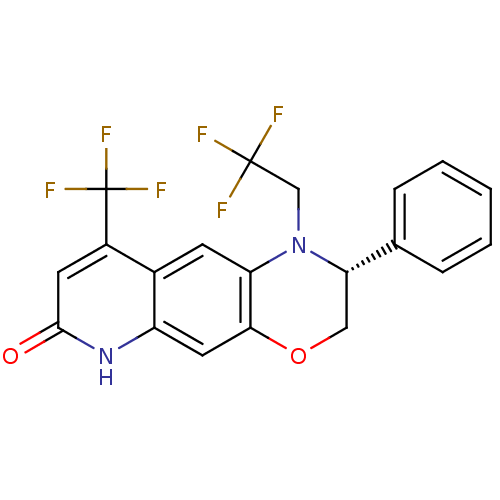
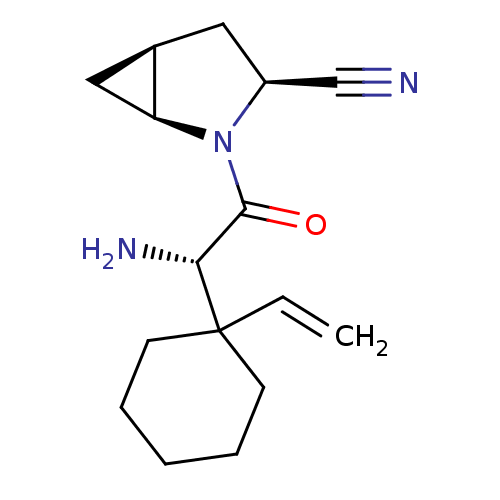
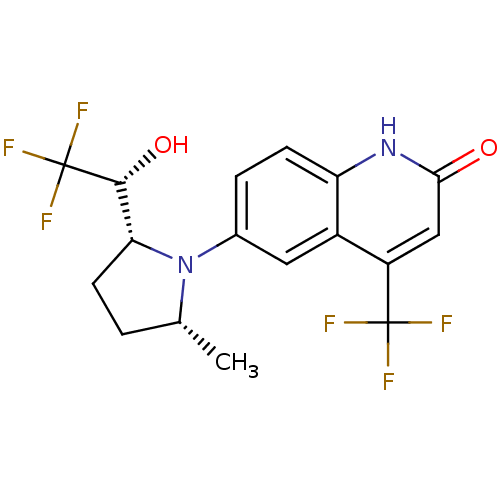
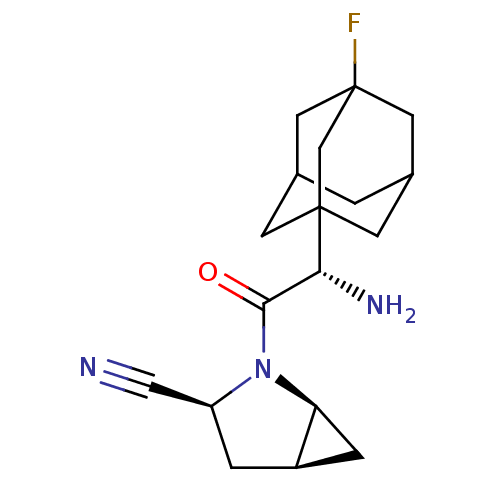
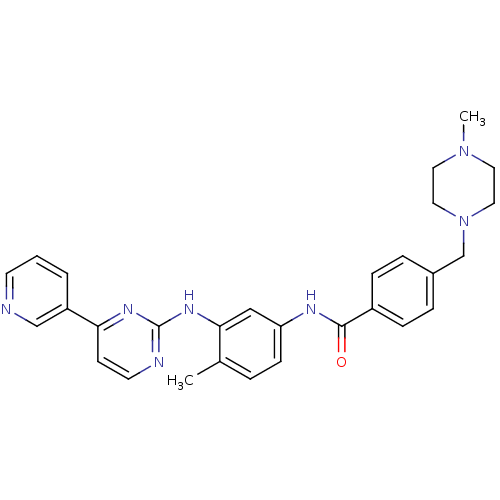
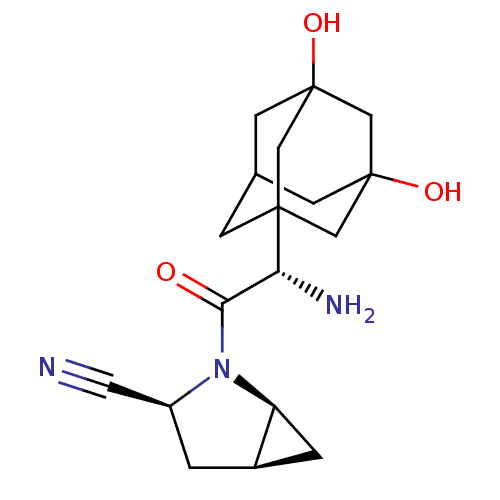
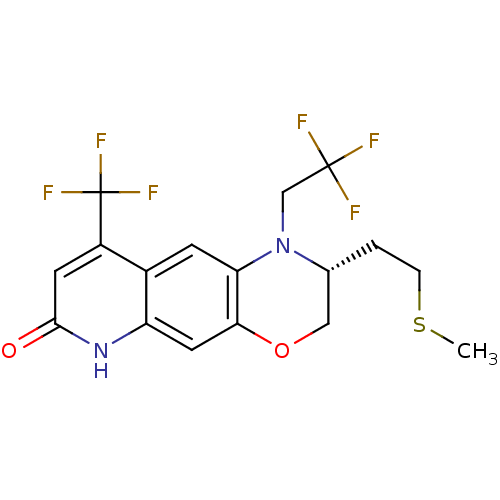
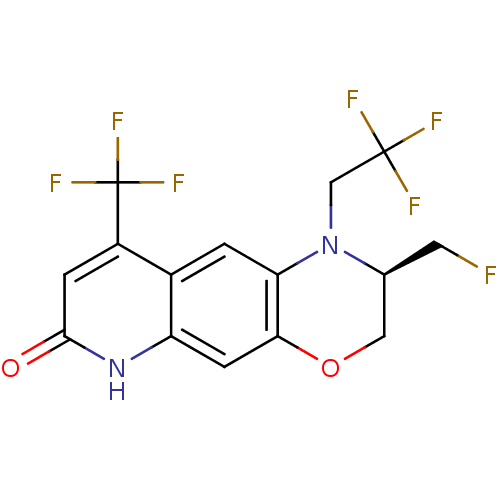
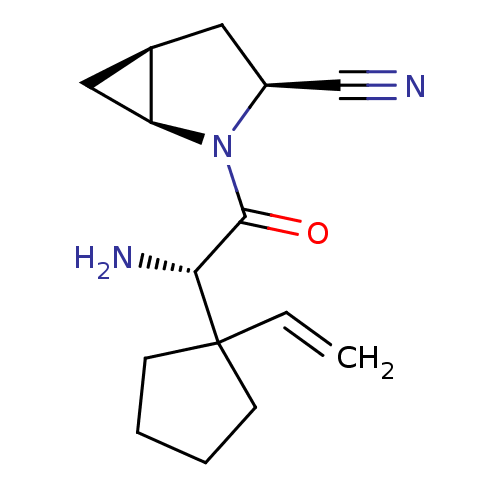
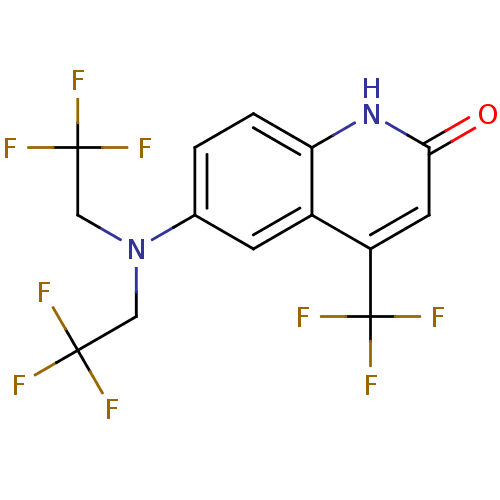
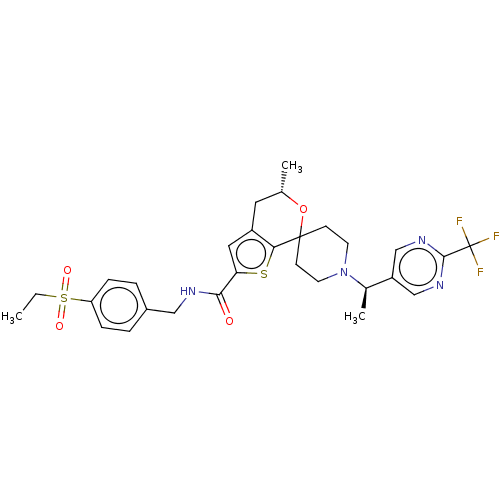
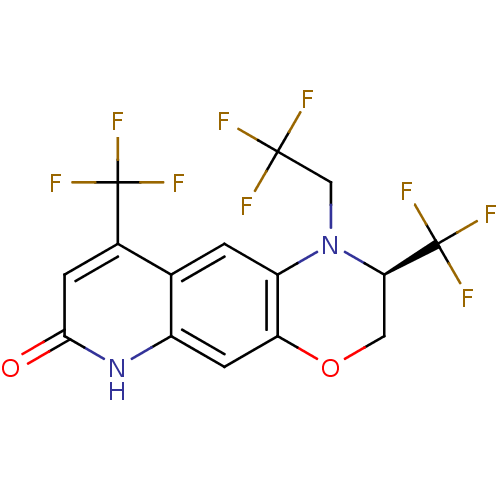
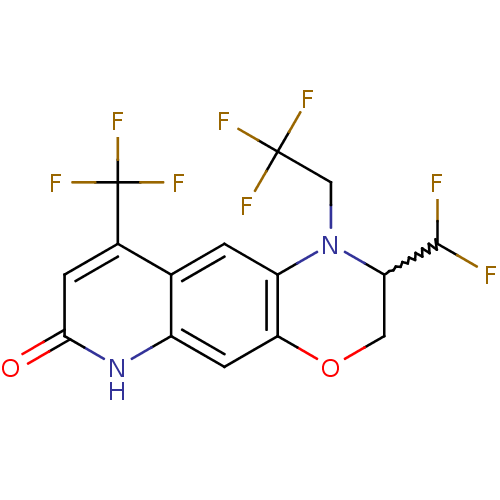
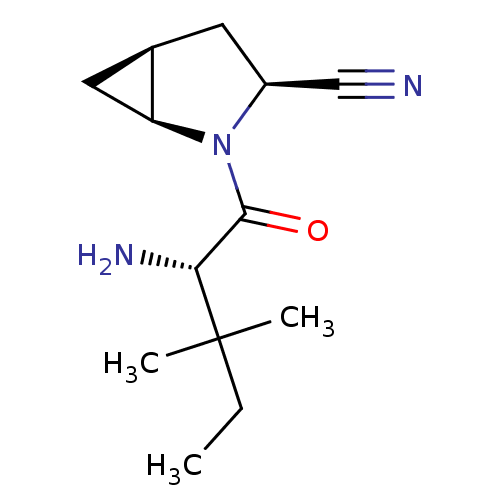
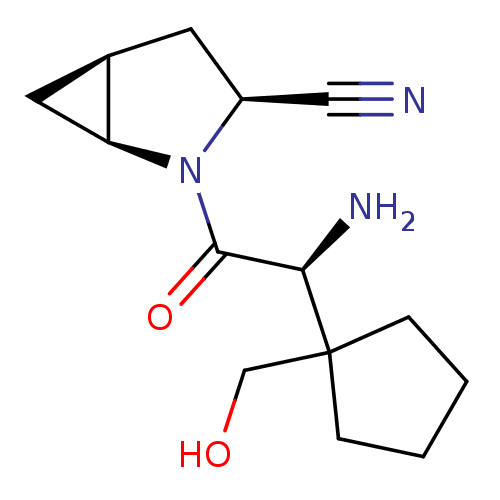
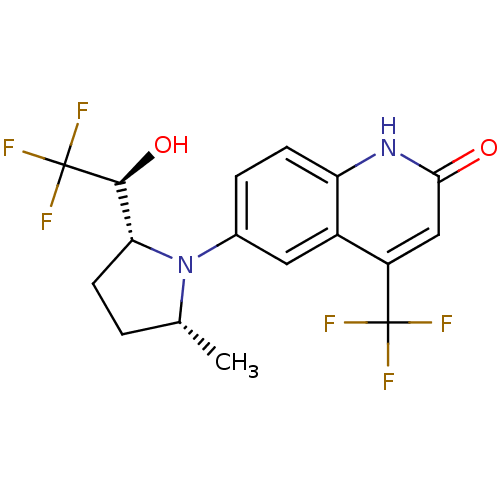
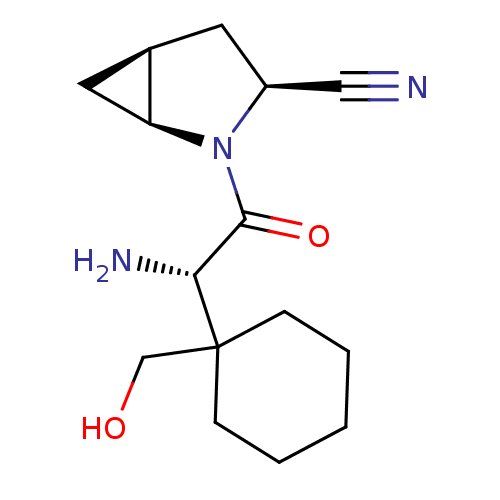
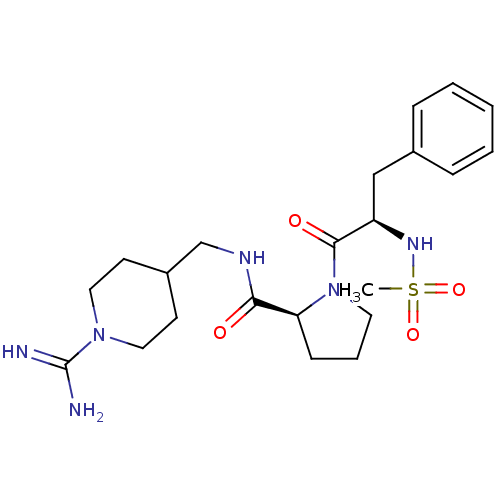
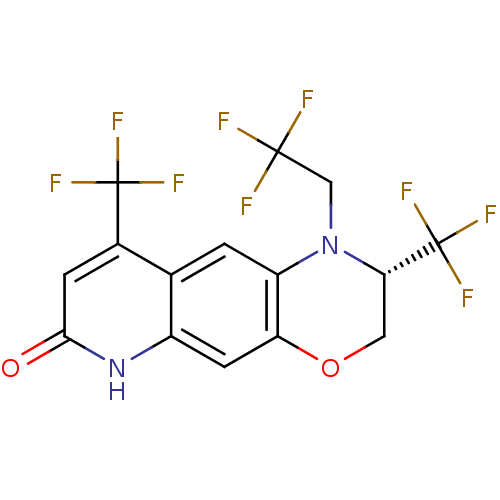
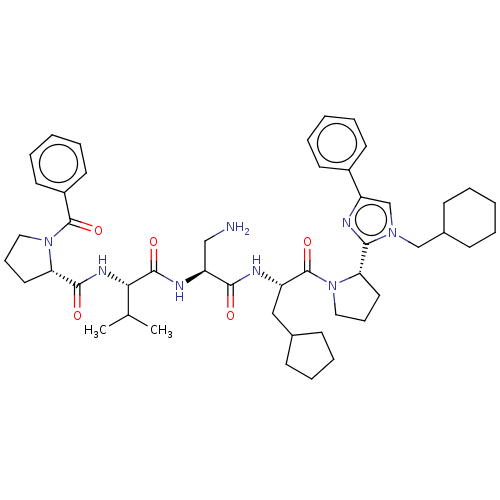
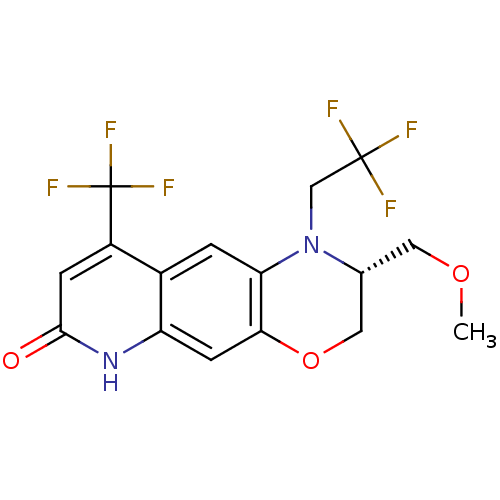
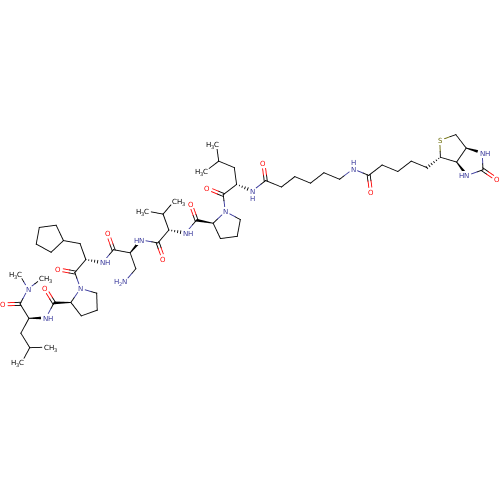
Affinity DataKi: 0.0160nMAssay Description:Inhibition of HIV1 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole EC50: 5.10nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Inhibition of HIV1 wild type protease using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Inhibition of HIV1 protease I47V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Competitive kinetic for thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora A kinaseMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 0.600nM ΔG°: -52.1kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Inhibition of HIV1 protease V82A mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of HIV1 protease L76V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 0.900nM ΔG°: -51.1kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.920nMAssay Description:Inhibition of HIV1 protease N88D mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Compound was tested for inhibitory activity against the binding of [3H]-spiperone to Dopamine receptor D2 in rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 1.40nM ΔG°: -50.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nM ΔG°: -52.4kJ/mole EC50: 7.10nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of Aurora B kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of Aurora B kinaseMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
TargetPlatelet-derived growth factor receptor alpha(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
TargetPlatelet-derived growth factor receptor alpha(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 2.10nM ΔG°: -49.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 3.90nM ΔG°: -47.5kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetEphrin type-B receptor 2(Homo sapiens (Human))
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Genomics Institute of The Novartis Research Foundation
Curated by PDSP Ki Database
Affinity DataKi: 4.20nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of Aurora C kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of Aurora C kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nM ΔG°: -49.5kJ/mole EC50: 0.200nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 5.5nM ΔG°: -46.7kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte...More data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.60nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 7.10nM ΔG°: -46.0kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 7.40nM ΔG°: -45.9kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
Affinity DataKi: 7.60nM ΔG°: -48.2kJ/mole EC50: 8.40nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
TargetDipeptidyl peptidase 4(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 8nM ΔG°: -45.7kJ/molepH: 7.4 T: 2°CAssay Description:Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Competitive kinetic for human alpha thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of N-Flu-DOT1L from MBP-tagged AF9 (unknown origin) (487 to 568 residues) expressed in Escherichia coli BL21(DE3) measured after 3 hrs b...More data for this Ligand-Target Pair
Affinity DataKi: 9.40nMAssay Description:Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of N-Flu-DOT1L from MBP-tagged AF9 (unknown origin) (487 to 568 residues) expressed in Escherichia coli BL21(DE3) measured after 3 hrs b...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)