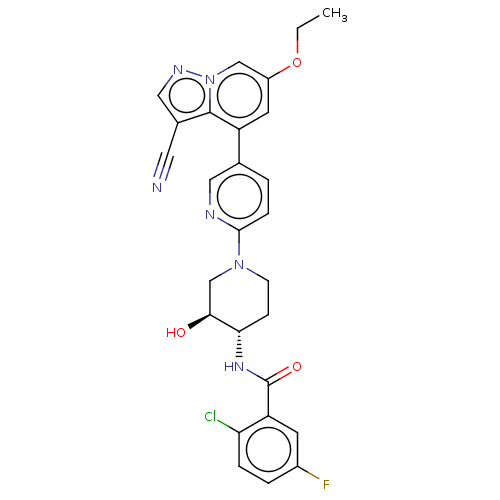
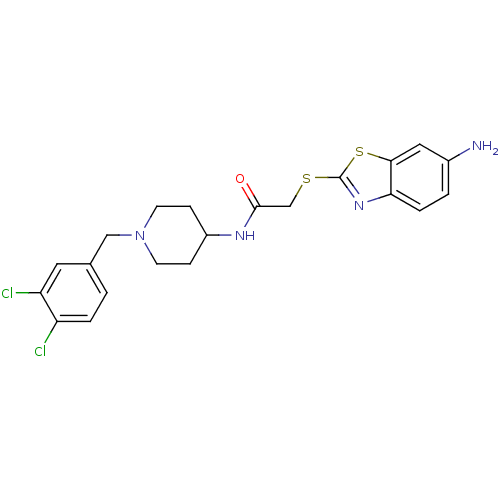
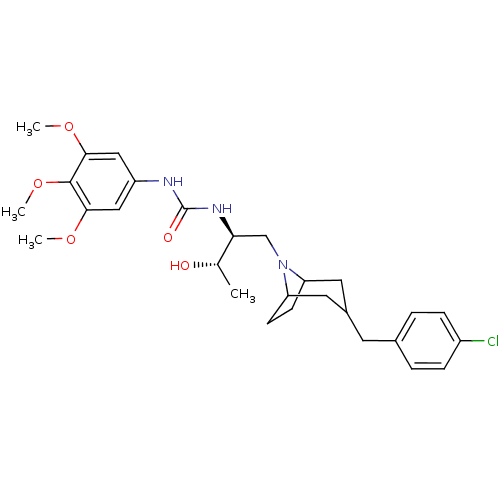
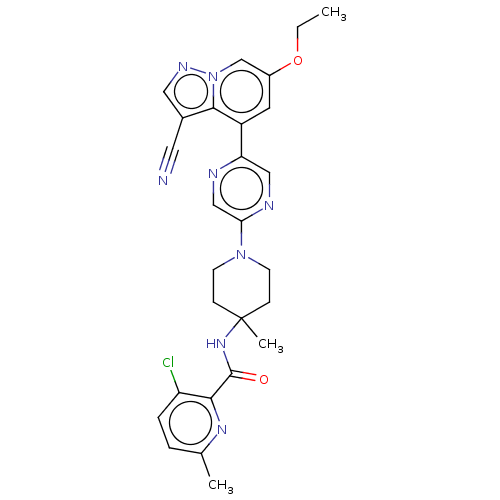
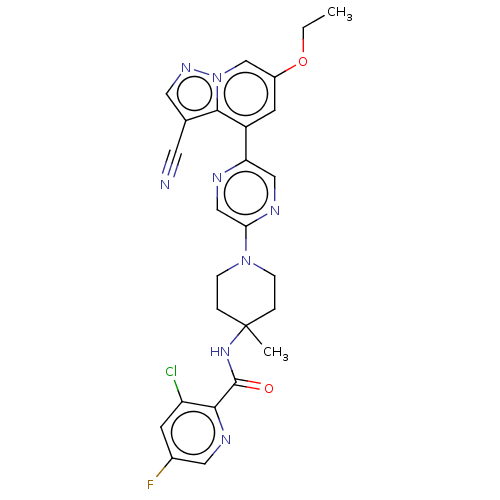
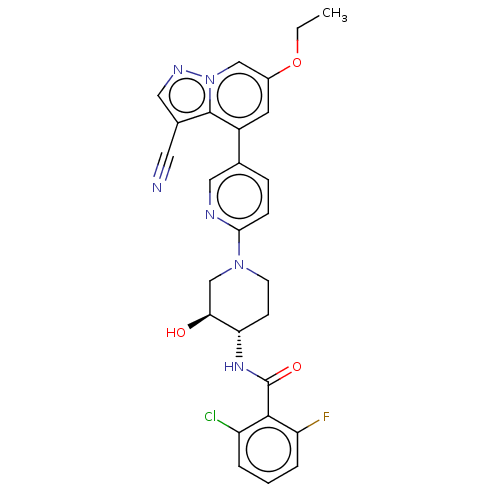
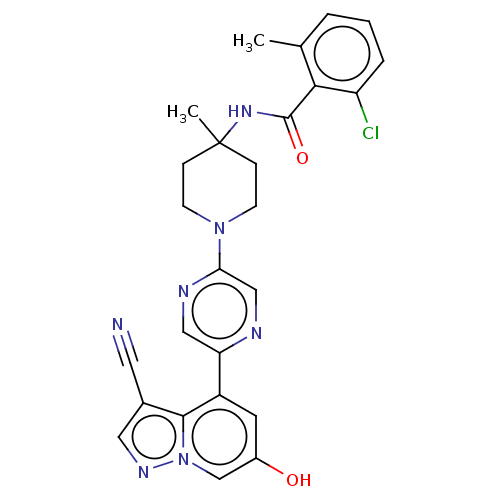
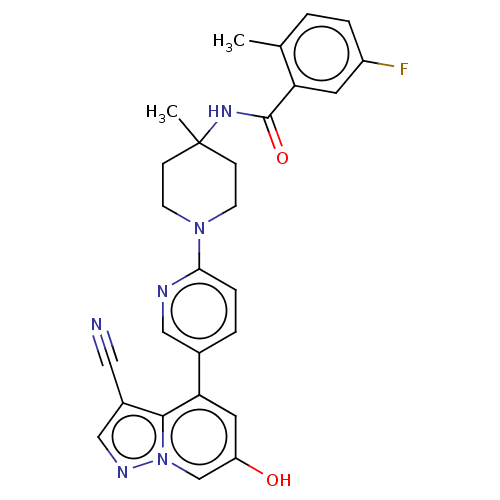
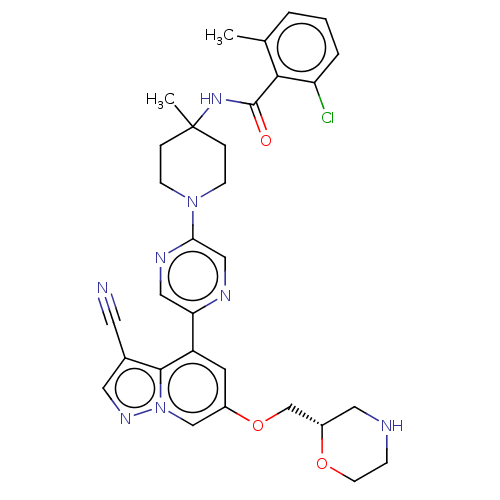
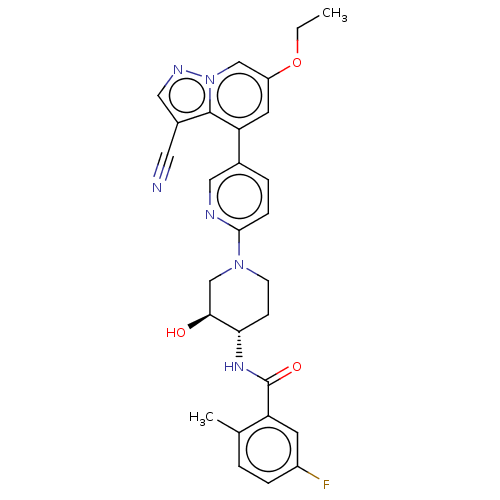
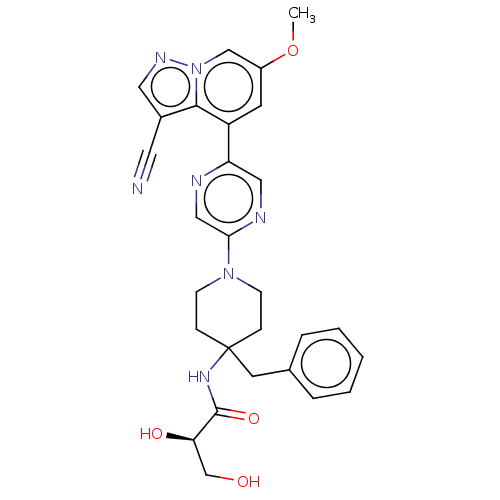
Affinity DataIC50: 0.158nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.251nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.316nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.631nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Binding affinity towards C-C chemokine receptor type 1More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 2nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 2nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 2nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 2nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 2nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptorMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE™-TK assay tec...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 3.70nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 4nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret (aa658-end)(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 4nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Array Biopharma
US Patent
Array Biopharma
US Patent
Affinity DataIC50: 4nMAssay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn...More data for this Ligand-Target Pair


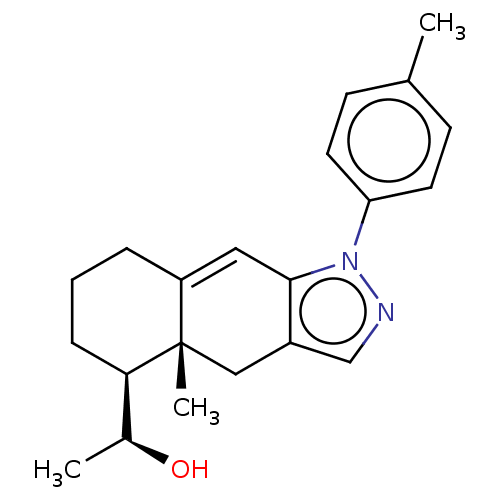
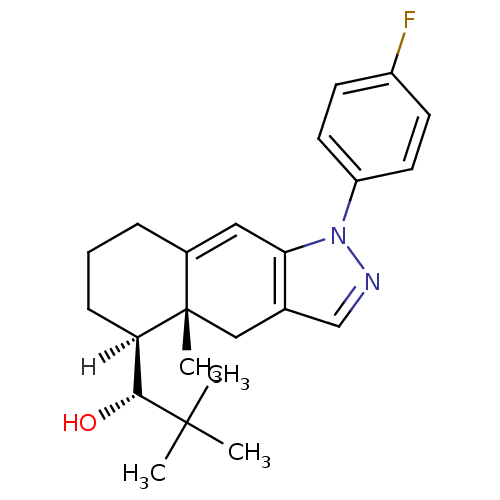
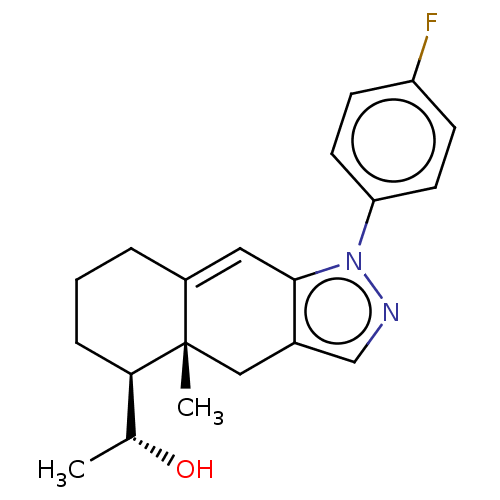
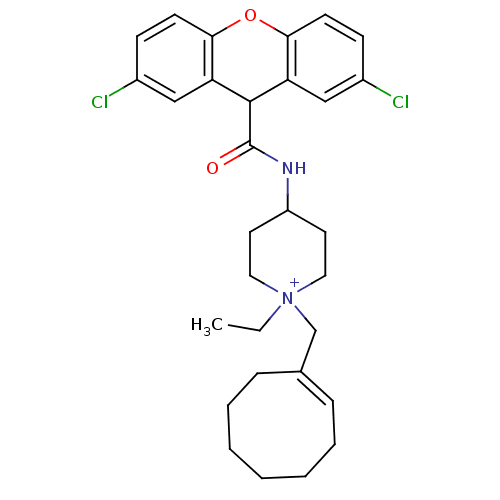
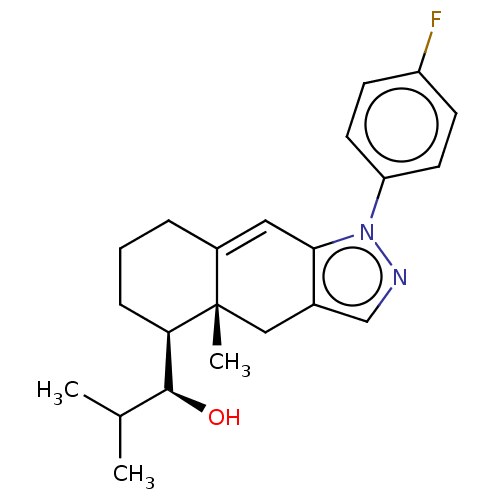
 3D Structure (crystal)
3D Structure (crystal)