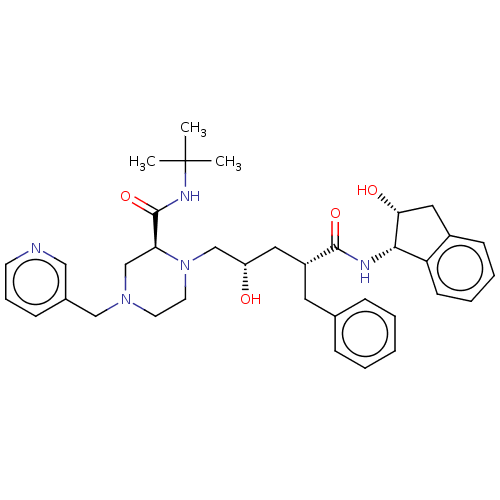
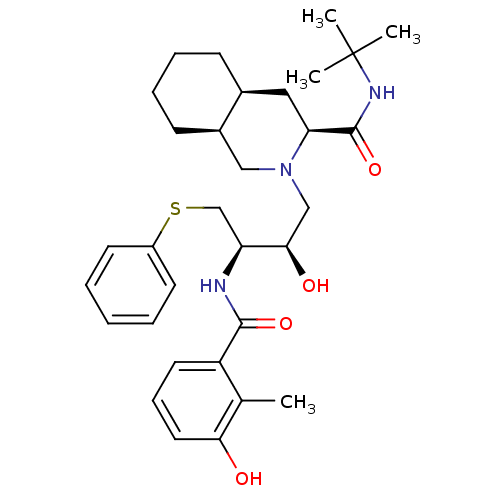
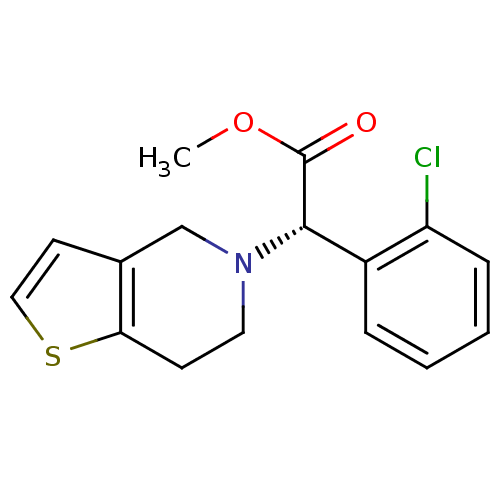
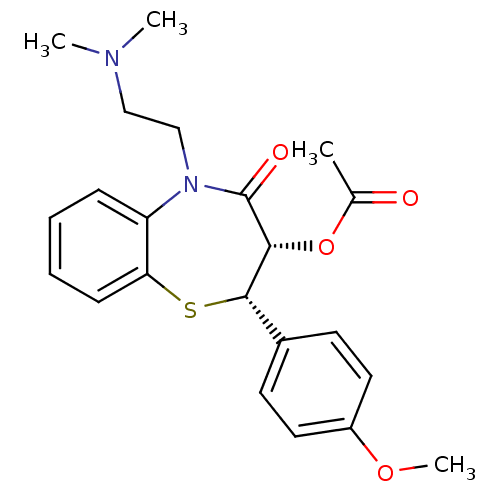
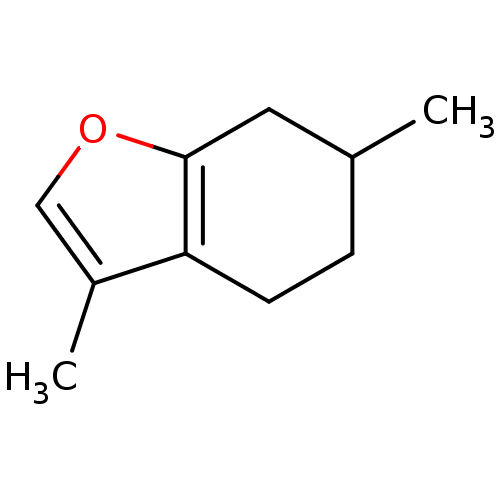
Affinity DataKi: 4nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A2 measured by 7-methoxyresorufin O-demethylation (MROD)More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Mechanism based inhibition of human cytochrome P450 1A2 measured by 7-methoxyresorufin O-demethylation (MROD)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A2 measured by 7-methoxyresorufin O-demethylation (MROD)More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A2More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-ethoxycoumarin O-deethylase activityMore data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Mechanism based inhibition of human cytochrome P450 1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4More data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A5More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by nifedipine oxidation, omeprazole 3-hydroxylation and omeprazole sulfoxydationMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-pentoxyresorufin O-deethylation activity (PROD)More data for this Ligand-Target Pair
Affinity DataKi: 142nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of human recombinant CYP2J2 expressed in baculovirus-infected Sf9 insect cellsMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 177nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Mechanism based inhibition of human cytochrome P450 2B6 measured by bupropion hydroxylation using human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Mechanism based inhibition of human cytochrome P450 1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by nifedipine oxidation, omeprazole 3-hydroxylation and omeprazole sulfoxydationMore data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:Mechanism based inhibition of dog cytochrome P450 CYP2B11 measured by Diazepam N1-demethylation using recombinant enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 373nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 399nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by N-demethylation of erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 460nMAssay Description:Mechanism based inhibition of dog cytochrome P450 CYP2B11 measured by Diazepam N1-demethylation using liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Mechanism based inhibition of human cytochrome P450 2B6 using human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A1 measured by 7-ethoxyresorufin O-deethylation (EROD)More data for this Ligand-Target Pair
Affinity DataKi: 570nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Mechanism based inhibition of human cytochrome P450 2A6 measured by coumarin 7-hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Mechanism based inhibition of human cytochrome P450 2B6 measured by bupropion hydroxylation using recombinant CYP2B6More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Mechanism based inhibition of human cytochrome P450 2A6 measured by coumarin 7-hydroxylation using a recombinant systemMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Mechanism based inhibition of human cytochrome P450 2B6 measured by 7-EFC O-deethylationMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-pentoxyresorufin O-deethylation activity (PROD)More data for this Ligand-Target Pair
Affinity DataKi: 840nMAssay Description:Mechanism based inhibition of human cytochrome P450 2A6 measured by coumarin 7-hxdroxylation using purified P450More data for this Ligand-Target Pair
Affinity DataKi: 870nMAssay Description:Mechanism based inhibition of rat cytochrome P450 CYP1A2 measured by 7-methoxyresorufin O-demethylation (MROD)More data for this Ligand-Target Pair




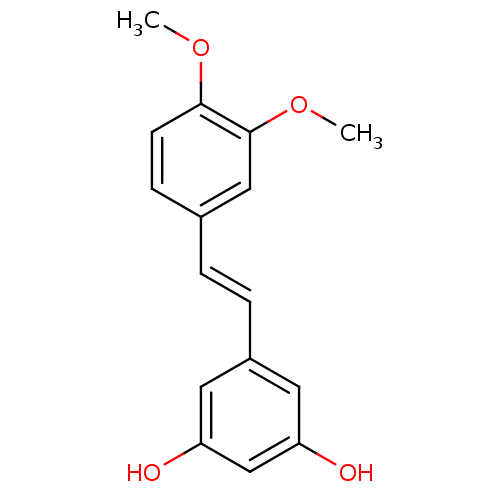
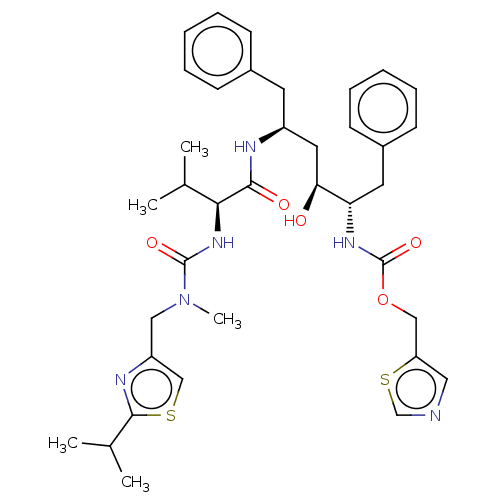
 3D Structure (crystal)
3D Structure (crystal)