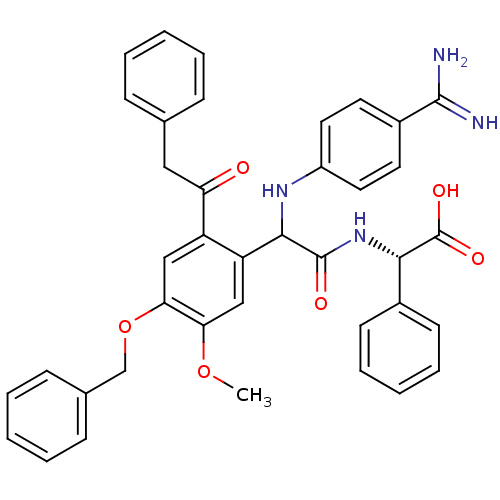
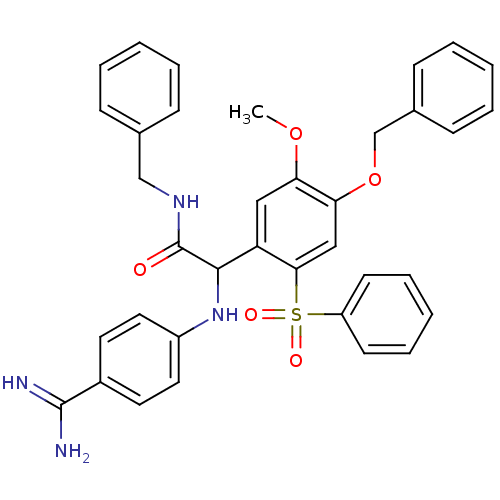
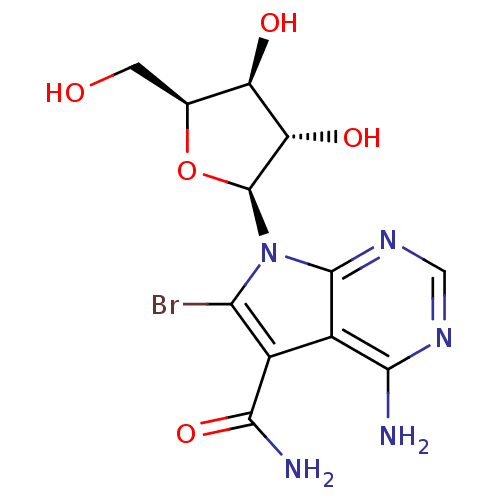
Affinity DataKi: 2nM ΔG°: -49.2kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
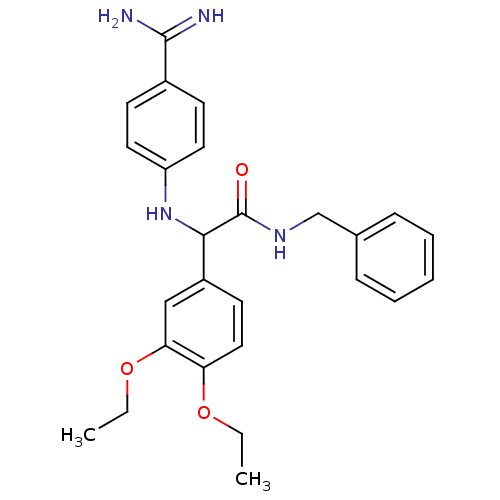
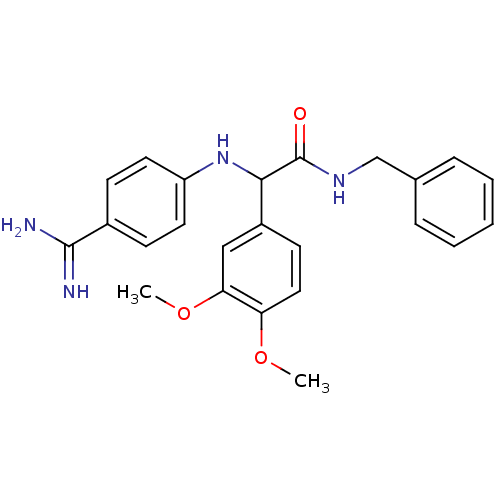
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
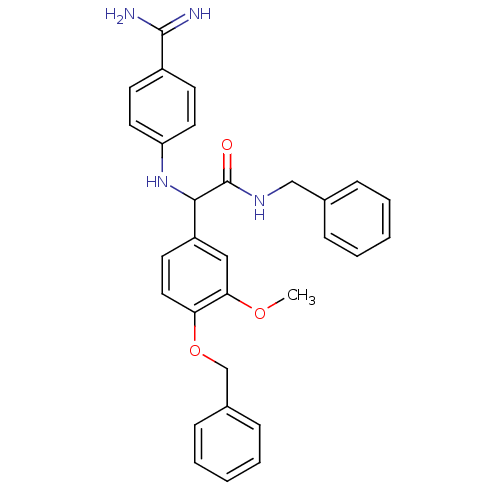
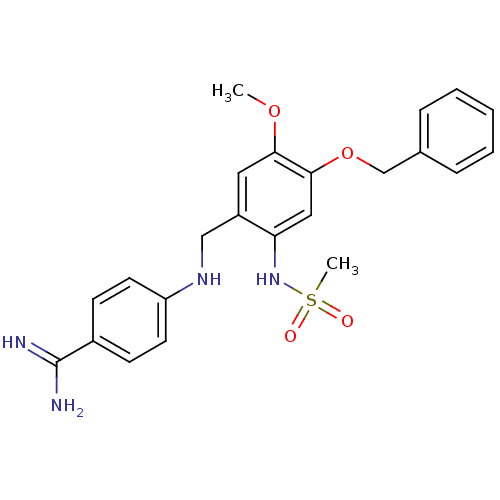
Affinity DataKi: 7nM ΔG°: -46.1kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
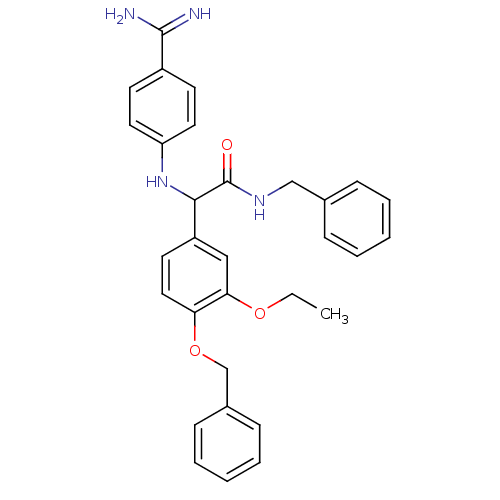
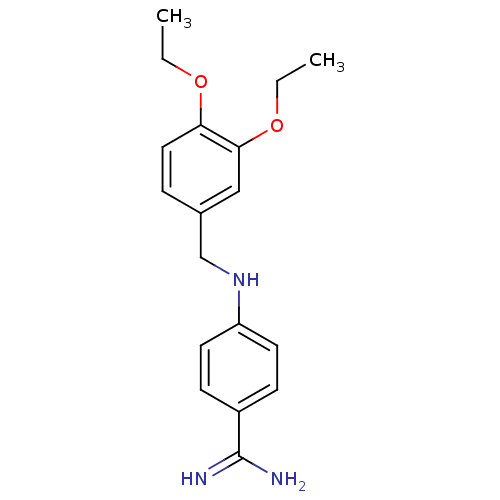
Affinity DataKi: 35nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 40nM ΔG°: -41.8kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -41.6kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 48nM ΔG°: -41.4kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 130nM ΔG°: -38.9kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 160nM ΔG°: -38.4kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 220nM ΔG°: -37.6kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 350nM ΔG°: -36.5kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 380nM ΔG°: -36.3kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 460nM ΔG°: -35.8kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 570nM ΔG°: -35.3kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 580nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -33.9kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nM ΔG°: -30.4kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 6.40E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.24E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >7.40E+4nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Jilin University
Curated by ChEMBL
Jilin University
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of CDK1/Cyclin B in human HeLa cell extracts using histone H1 as substrate preincubated for 30 mins before substrate addition measured aft...More data for this Ligand-Target Pair
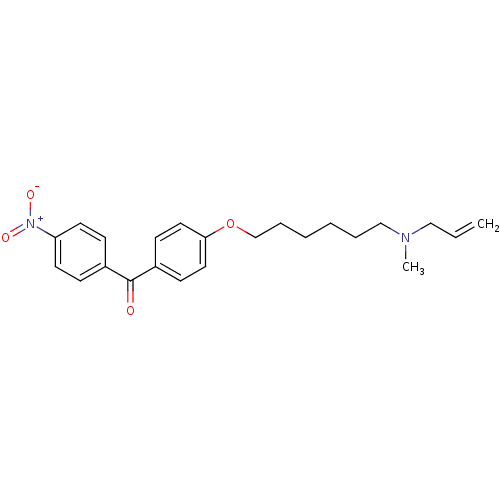
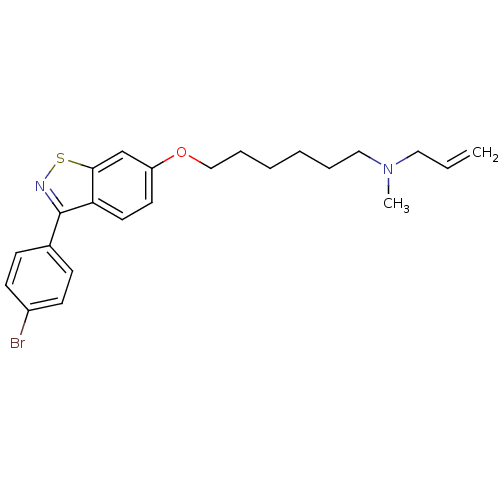
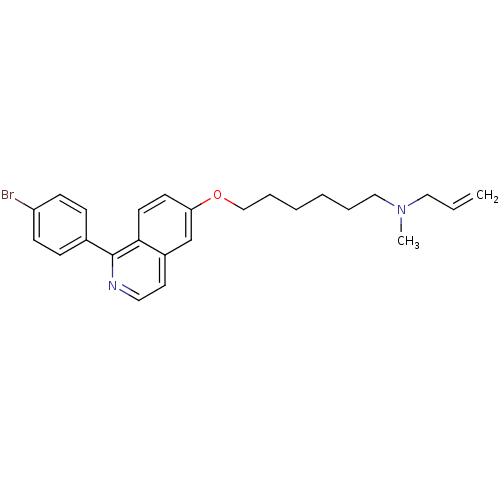
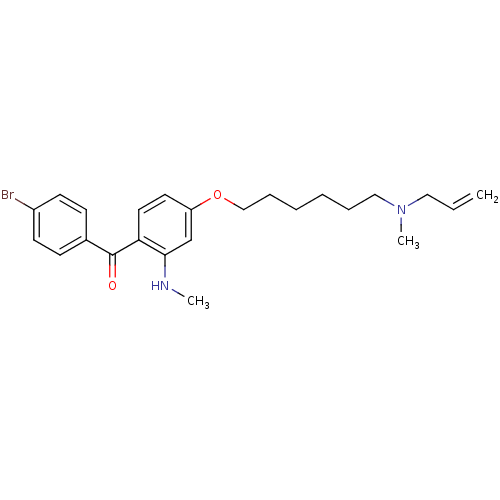
Affinity DataIC50: 1.90nMAssay Description:In vitro inhibition of human 2,3-oxidosqualene cyclase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:In vitro inhibition of human 2,3-oxidosqualene cyclase.More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:In vitro inhibition of human 2,3-oxidosqualene cyclase.More data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:In vitro inhibition of human 2,3-oxidosqualene cyclase.More data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:In vitro inhibition of human 2,3-oxidosqualene cyclase.More data for this Ligand-Target Pair

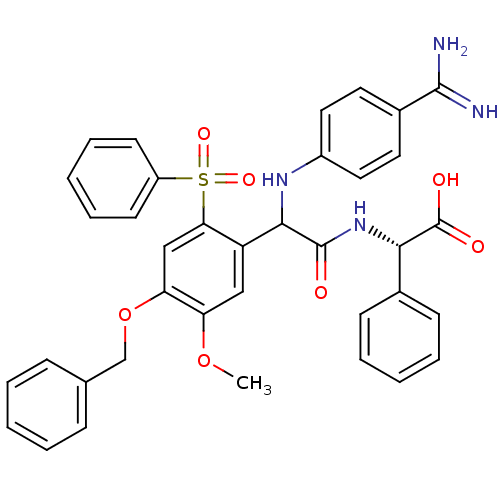
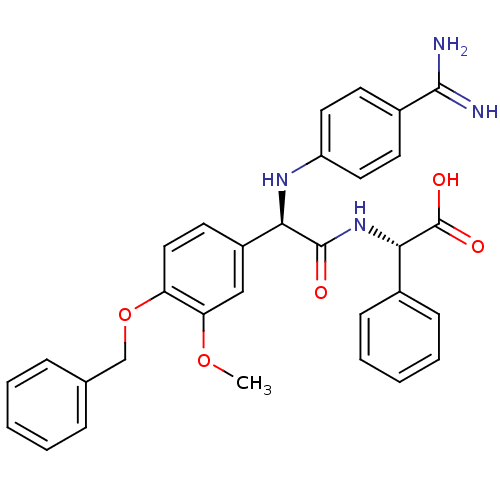
 3D Structure (crystal)
3D Structure (crystal)