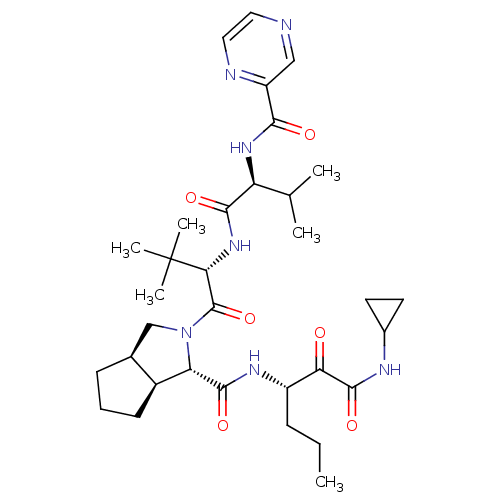
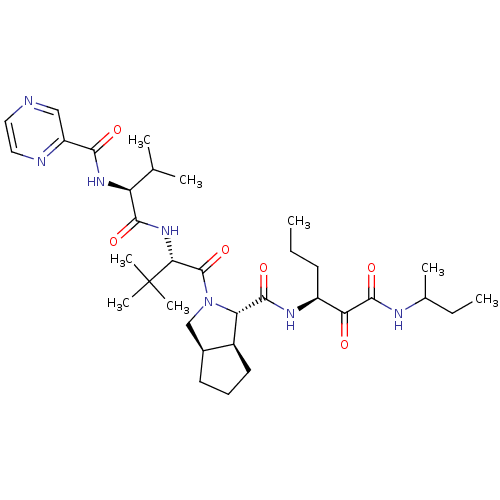
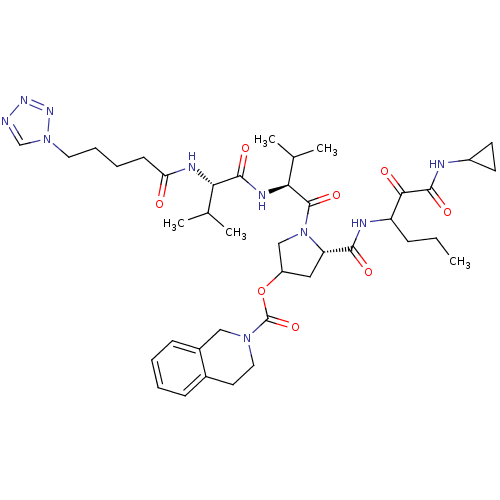
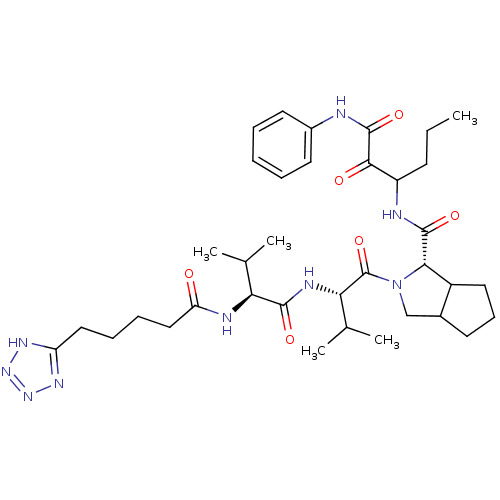
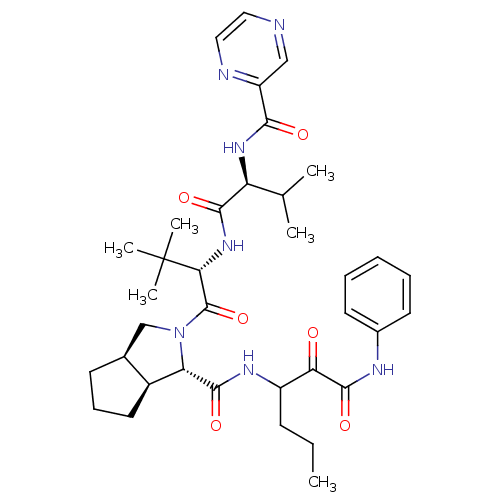
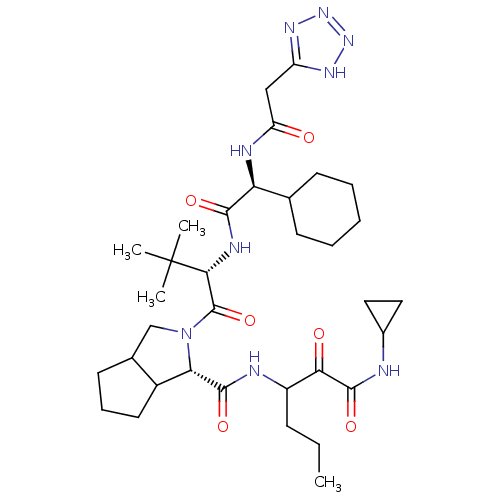
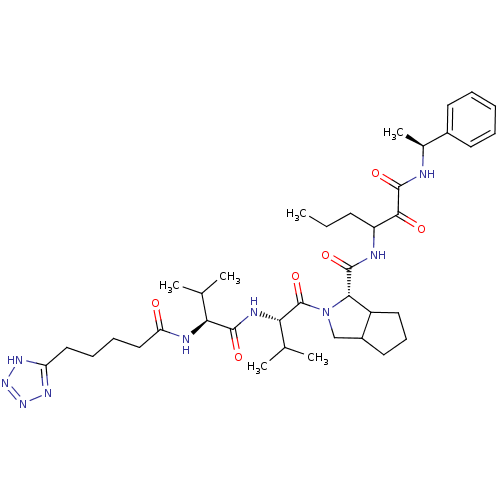
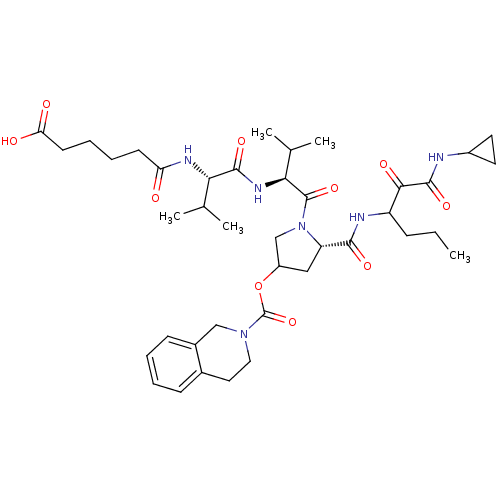
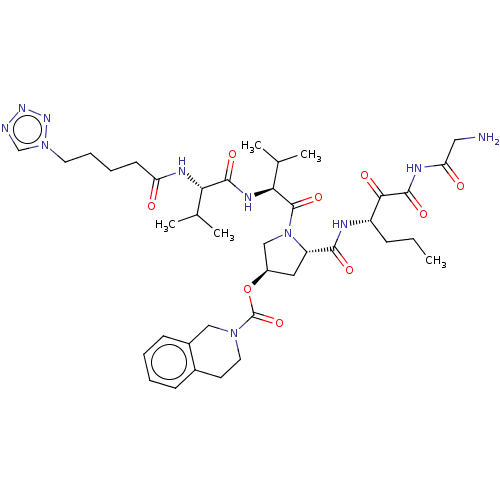
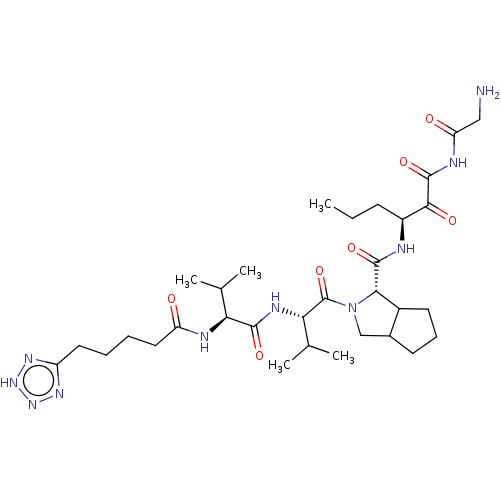
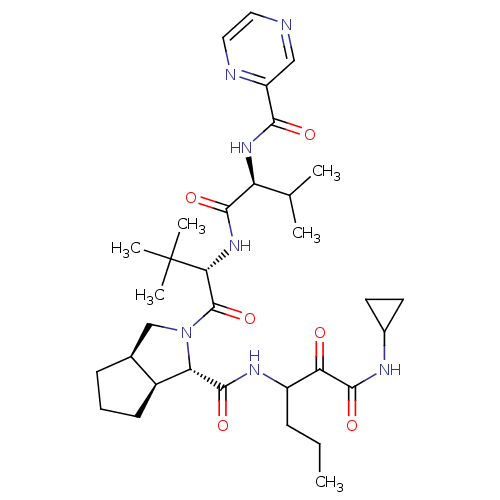
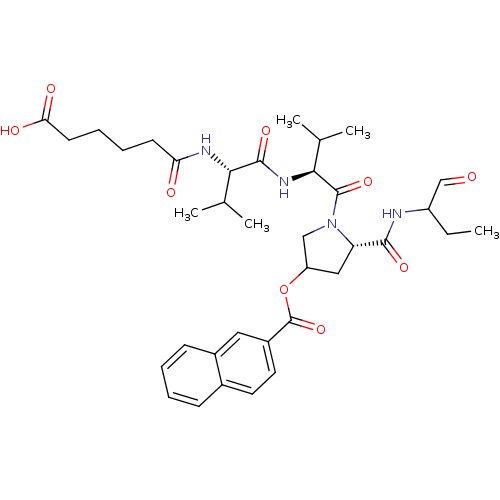
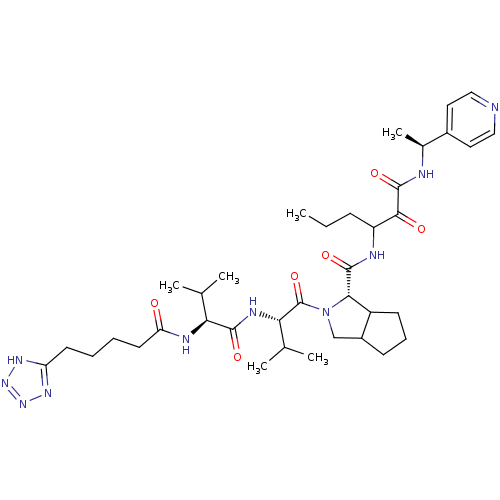
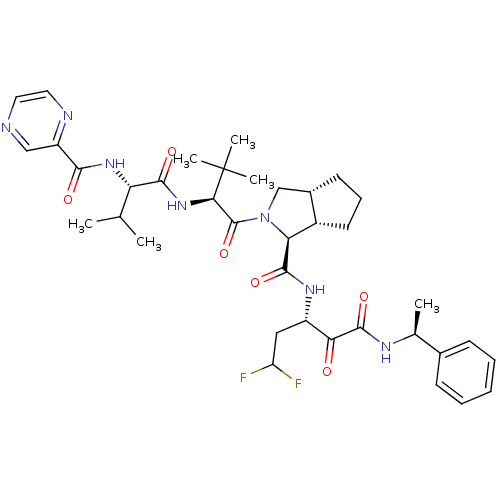
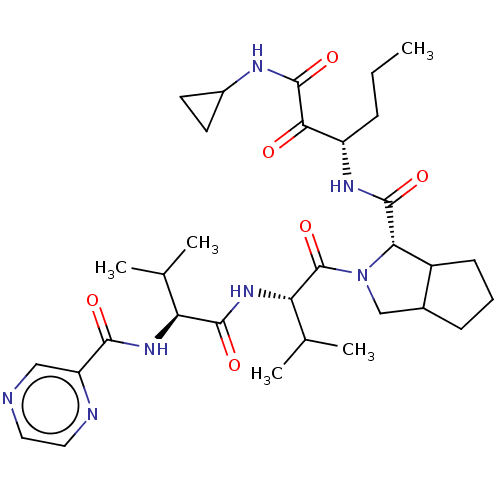
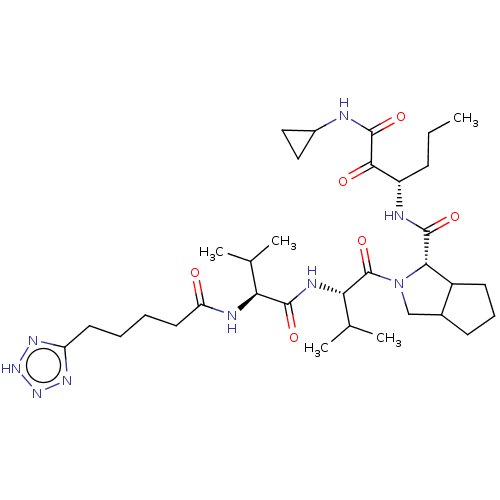
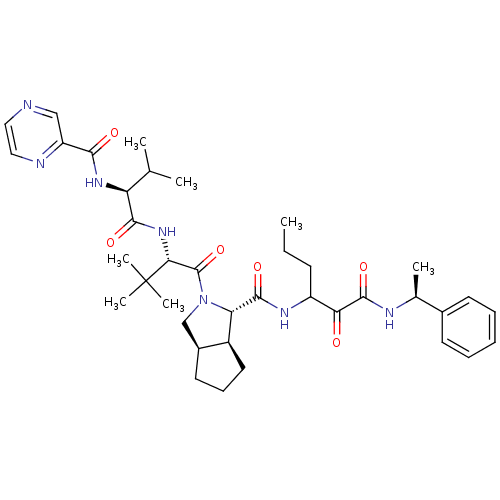
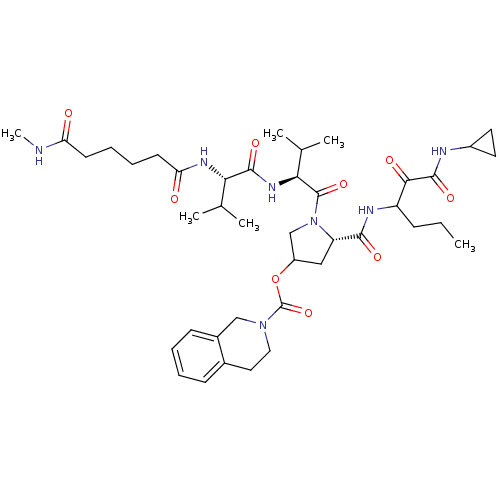
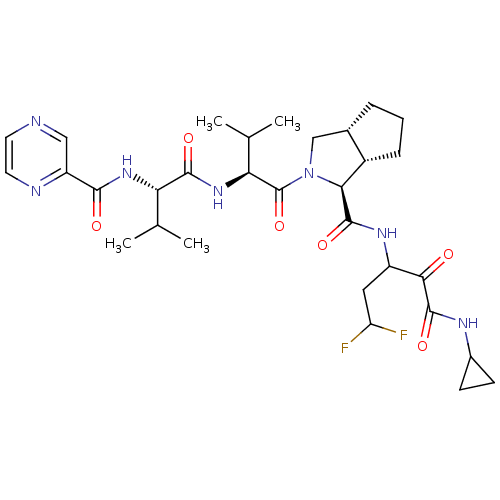
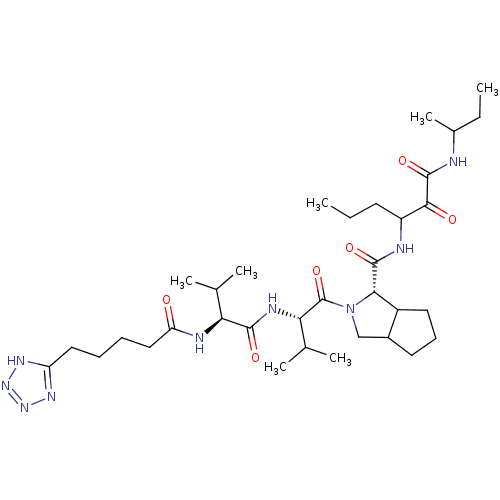
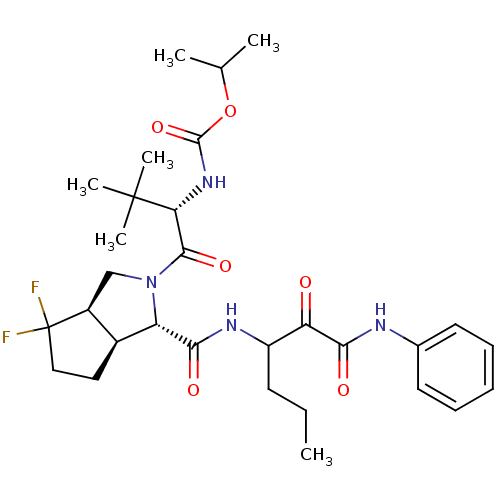
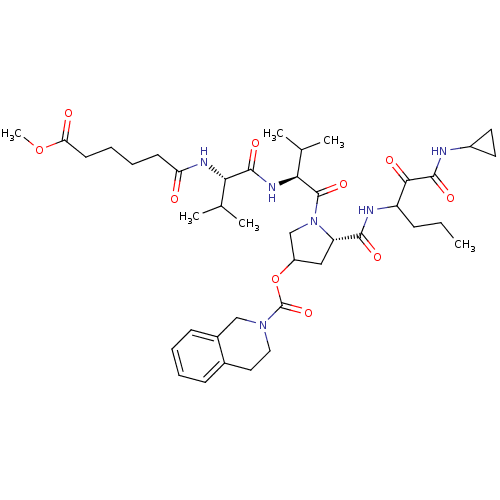
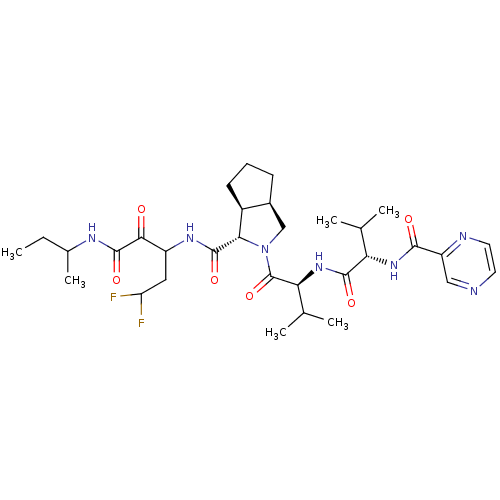
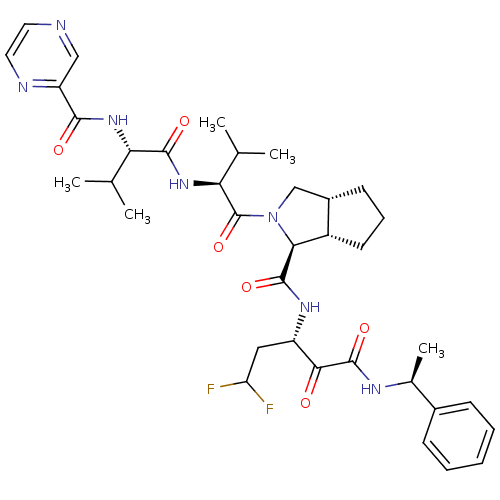
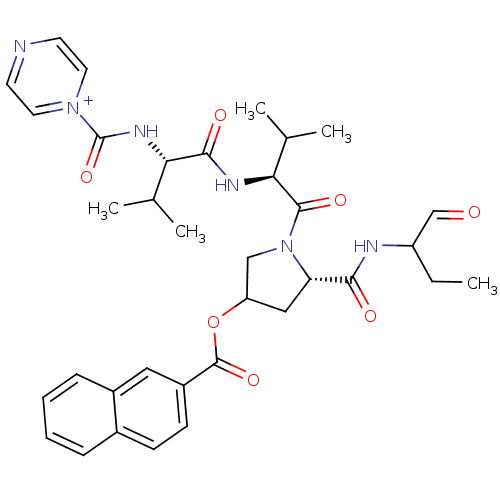
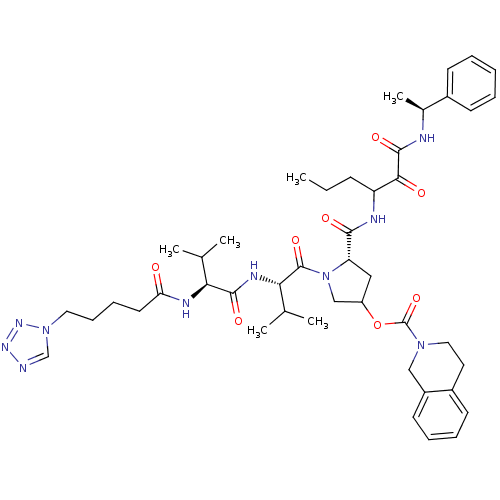
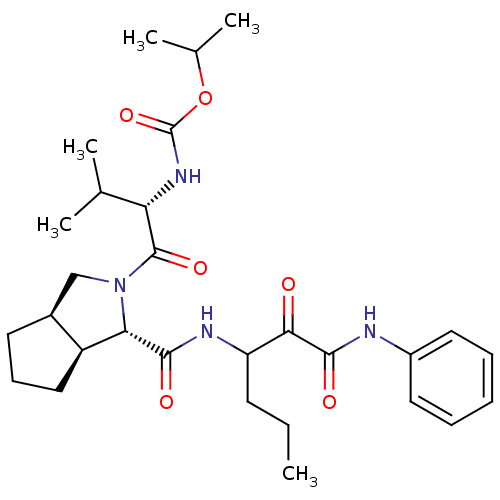
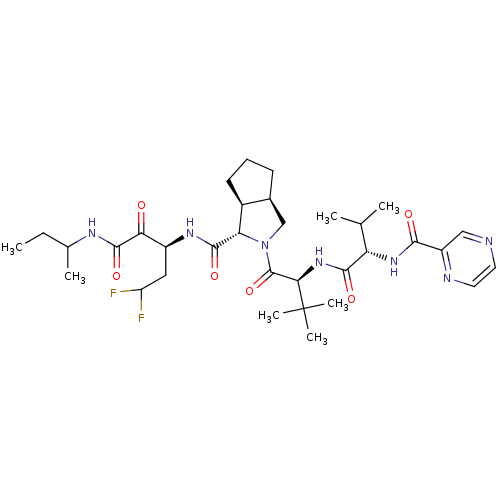
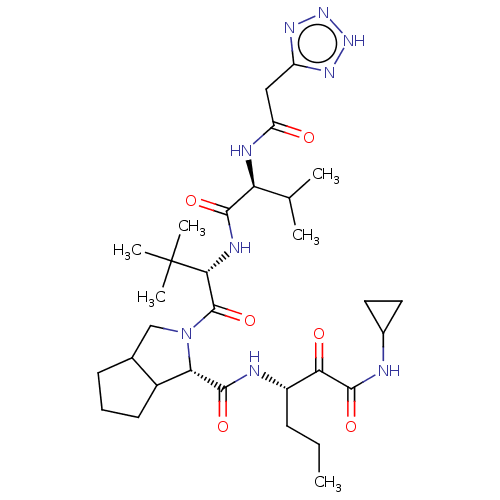
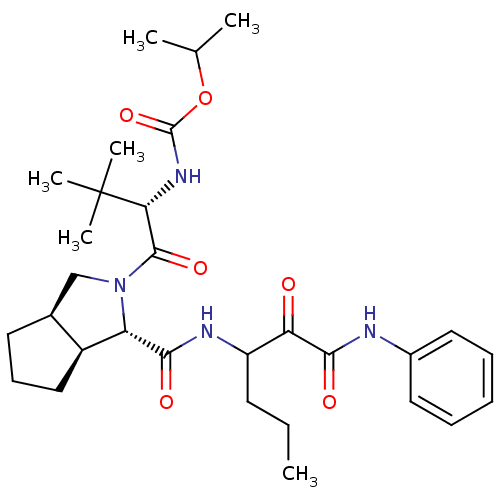
Affinity DataKi: 1.60nMAssay Description:Inhibitory activity of the compound against cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibitory activity of the compound against cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity of the compound against cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibitory activity of the compound against cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibitory activity of the compound against cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Binding affinity towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Inhibitory activity of the compound against cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibitory activity of the compound against cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Inhibitory activity of the compound against cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Inhibitory activity of the compound against cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Inhibitory activity of the compound against ChymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 74nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 84nMAssay Description:Binding affinity towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 84nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 112nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 123nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 123nMAssay Description:Cytotoxic activity in rat liver Huh-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 154nMAssay Description:Inhibitory activity of the compound against cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Binding affinity towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 193nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibitory activity of the compound against HCV protease using replicon assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 229nMAssay Description:Inhibitory activity of the compound against cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 248nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:Binding affinity towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 271nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 303nMAssay Description:Inhibitory activity of the compound against ChymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 380nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity of the compound towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 404nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Binding affinity towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Inhibitory activity of the compound against HCV protease using replicon assay in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 492nMAssay Description:Inhibitory activity of the compound against cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 583nMAssay Description:Inhibitory activity of the compound against ChymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 683nMAssay Description:Inhibitory activity of the compound against cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 808nMAssay Description:Inhibitory potency against HCV NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 820nMAssay Description:Binding affinity towards Protease using PNA assay in ratsMore data for this Ligand-Target Pair