Affinity DataIC50: 2.25E+3nMAssay Description:Inhibition of BChE (unknown origin) using acetylcholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.32E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.82E+3nMAssay Description:Inhibition of BChE (unknown origin) using acetylcholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.83E+3nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.76E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylcholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.66E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylcholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.45E+4nMAssay Description:Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.44E+4nMAssay Description:Inhibition of tyrosinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.51E+4nMAssay Description:Inhibition of tyrosinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 7.85E+4nMAssay Description:Inhibition of tyrosinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+5nMAssay Description:Inhibition of AChE (unknown origin) using acetylcholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+5nMAssay Description:Inhibition of BChE (unknown origin) using acetylcholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair

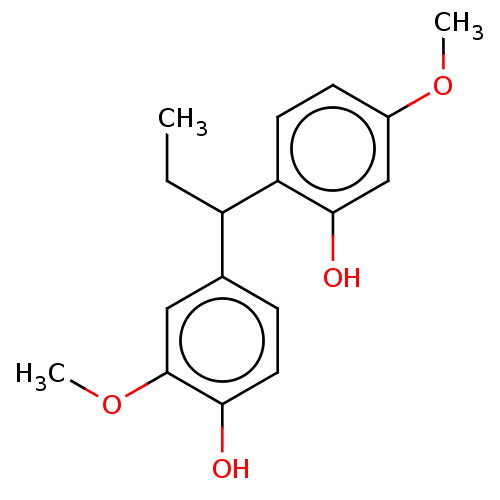
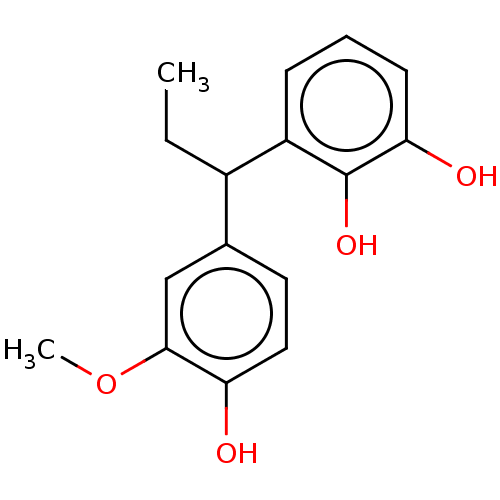
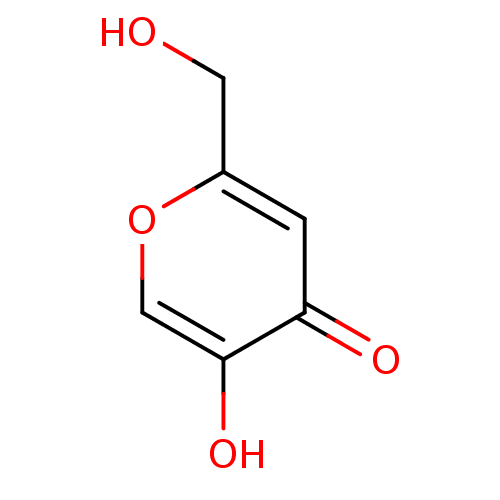
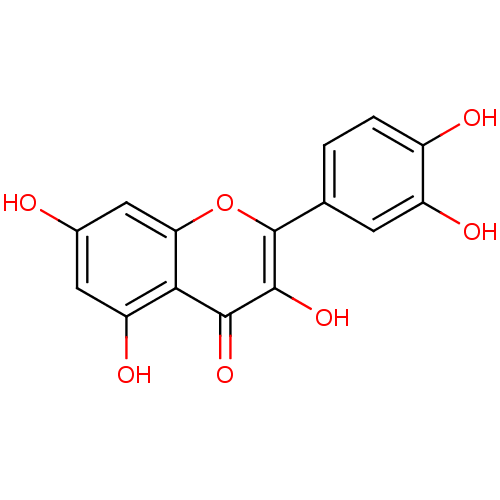

 3D Structure (crystal)
3D Structure (crystal)