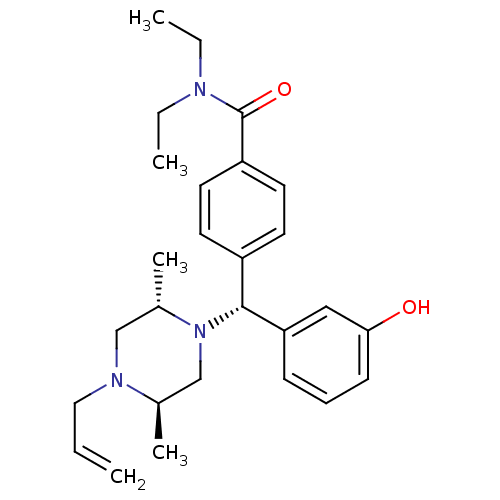
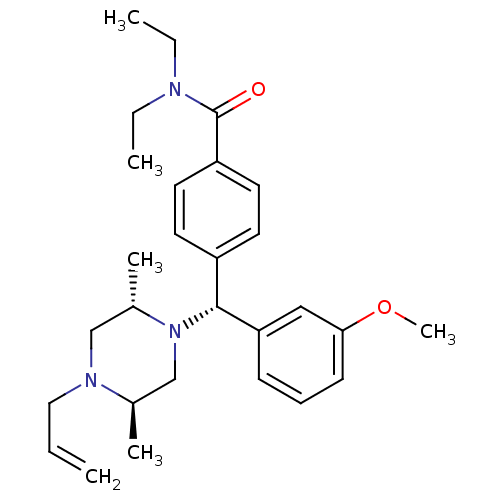
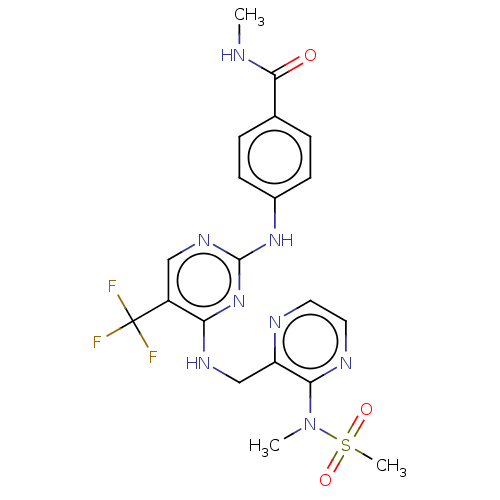
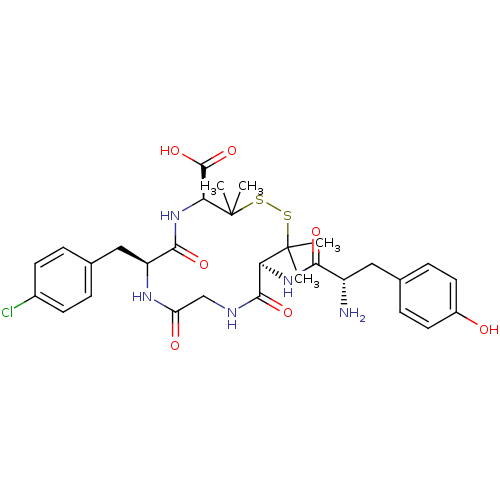
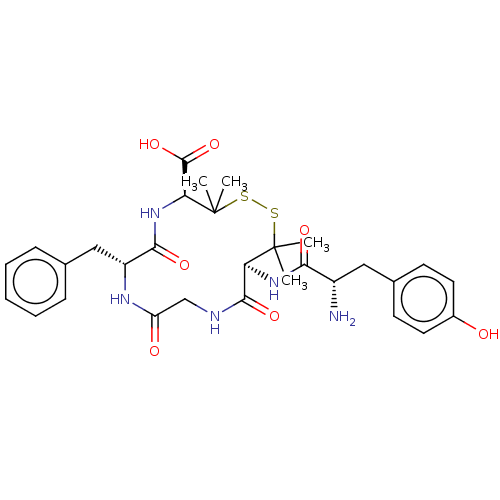
Affinity DataKi: <4nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
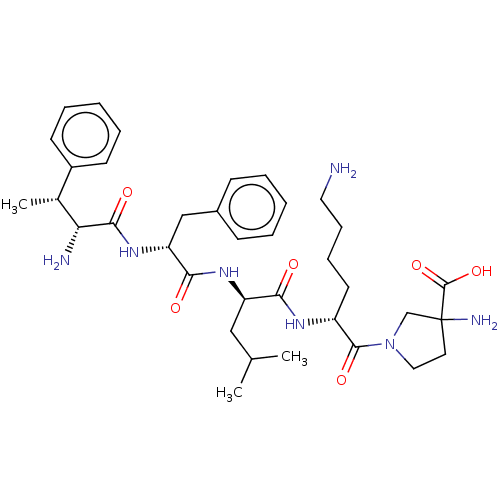
Affinity DataKi: 5nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
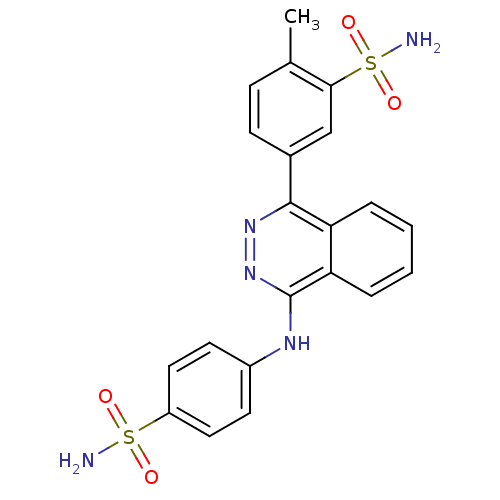
Affinity DataKi: 6nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
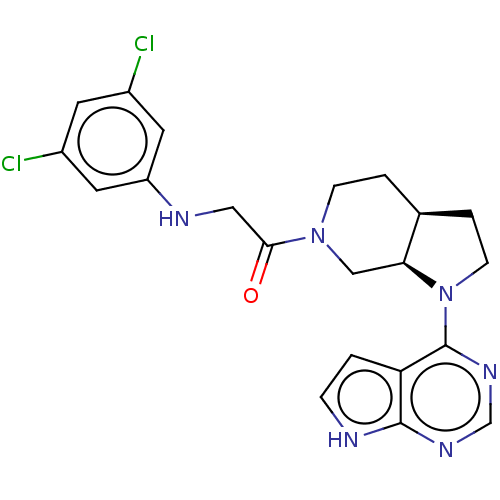
Affinity DataKi: 9nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 48nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Taigen Biotechnology
Taigen Biotechnology
Affinity DataKi: 53nM ΔG°: -41.5kJ/molepH: 7.5 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 57nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Taigen Biotechnology
Taigen Biotechnology
Affinity DataKi: 58nM ΔG°: -41.3kJ/molepH: 7.5 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 66nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 66nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 83nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 105nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 114nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 144nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 188nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 191nMAssay Description:The compound was evaluated for the binding affinity towards delta-opioid receptor by displacement of [3H]p-CI-DPDPE radioligand from mouse vas defere...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 193nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 203nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 386nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Taigen Biotechnology
Taigen Biotechnology
Affinity DataKi: 660nM ΔG°: -35.3kJ/molepH: 7.5 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Taigen Biotechnology
Taigen Biotechnology
Affinity DataKi: 2.26E+3nM ΔG°: -32.2kJ/molepH: 7.5 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
Taigen Biotechnology
Taigen Biotechnology
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.5 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse...More data for this Ligand-Target Pair
Affinity DataKi: 2.84E+4nMAssay Description:The compound was evaluated for the binding affinity towards mu-opioid receptor by displacement of [3H]DAMGO radioligand from guinea pig ileumMore data for this Ligand-Target Pair
Affinity DataIC50: 0.157nMAssay Description:The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma...More data for this Ligand-Target Pair
Affinity DataIC50: 0.158nMAssay Description:The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
East China University Of Science And Technology
Curated by ChEMBL
East China University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.253nMAssay Description:The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma...More data for this Ligand-Target Pair
Affinity DataIC50: 0.255nMAssay Description:The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma...More data for this Ligand-Target Pair
Affinity DataIC50: 0.294nMAssay Description:The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
East China University Of Science And Technology
Curated by ChEMBL
East China University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of full length carbonic anhydrase-2 in human erythrocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand.More data for this Ligand-Target Pair
Affinity DataIC50: 0.398nMAssay Description:The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
East China University Of Science And Technology
Curated by ChEMBL
East China University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 0.480nMAssay Description:Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
East China University Of Science And Technology
Curated by ChEMBL
East China University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 0.990nMAssay Description:Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin)More data for this Ligand-Target Pair
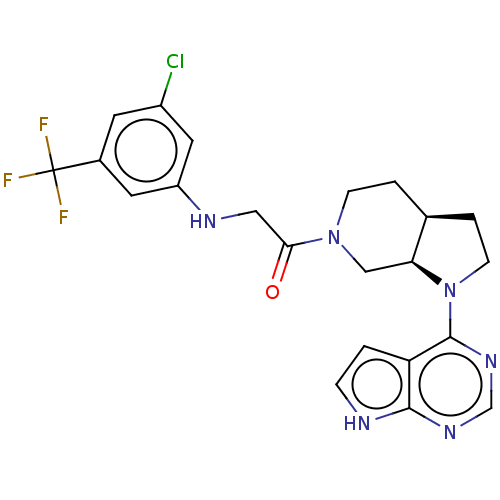
Affinity DataIC50: <1nMAssay Description:Inhibition of unphosphorylated BTK (unknown origin) in the presence of ATP by FRET assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
Affinity DataIC50: <1nMAssay Description:Inhibition of unphosphorylated BTK (unknown origin) in the presence of ATP by FRET assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
Affinity DataIC50: 1.06nMAssay Description:Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand.More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Binding affinity was measured against cloned human Opioid receptor delta 1 (wild-type,Wt)More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand.More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand.More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Binding affinity was measured against mutated human Opioid receptor delta 1 (W248L)More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
East China University Of Science And Technology
Curated by ChEMBL
East China University Of Science And Technology
Curated by ChEMBL
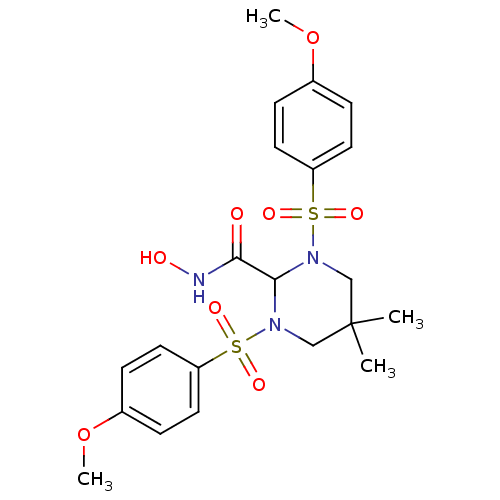
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human recombinant MMP9 catalytic domain incubated for 20 mins by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of unphosphorylated BTK (unknown origin) in the presence of ATP by FRET assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info

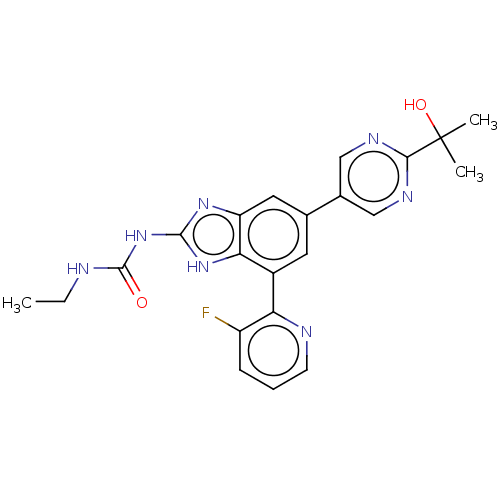
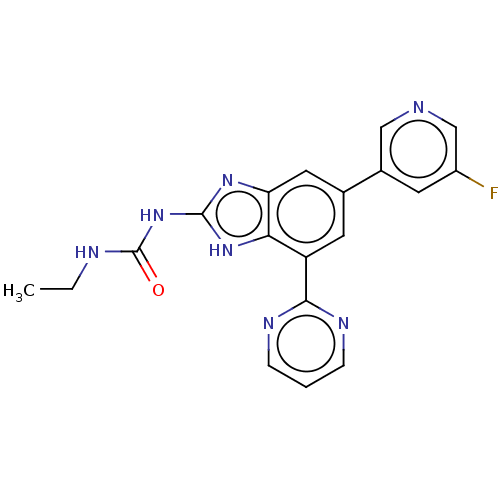
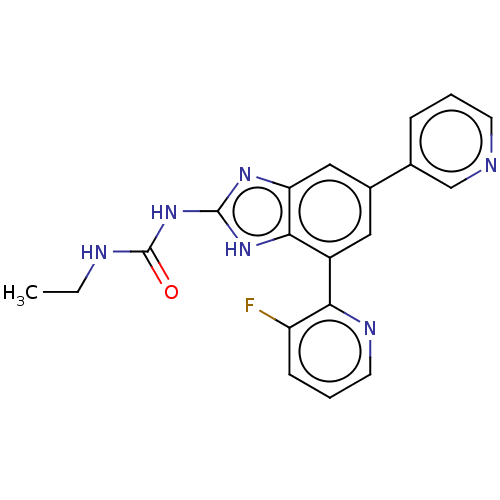
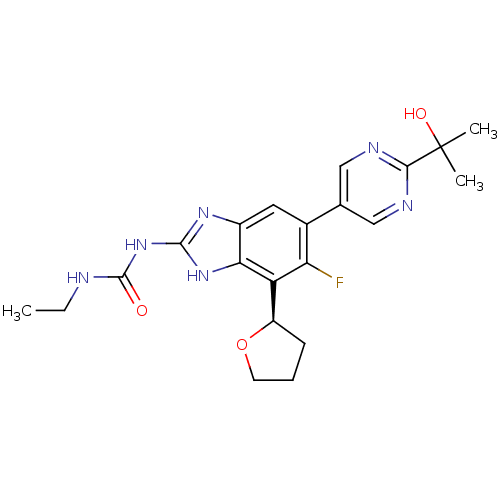
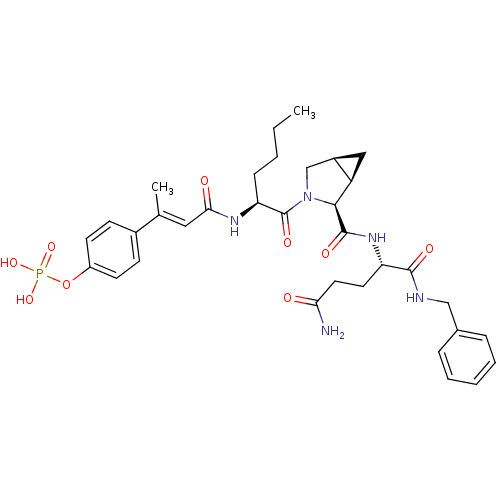
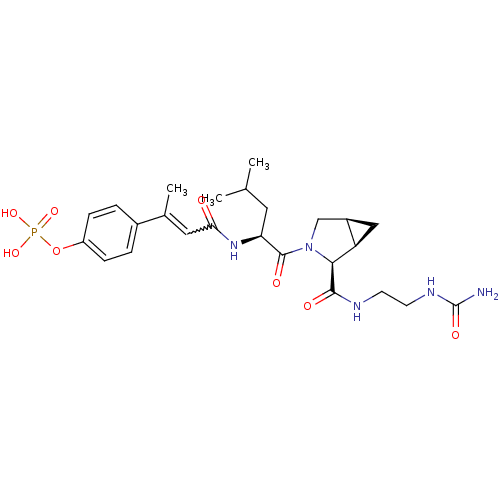
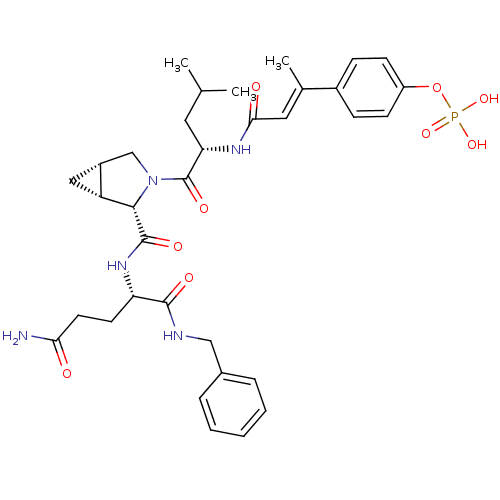
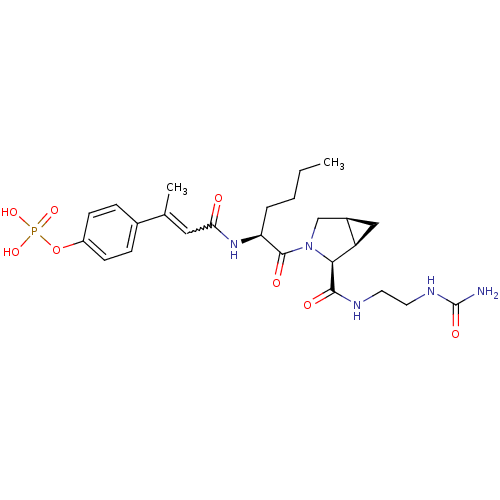
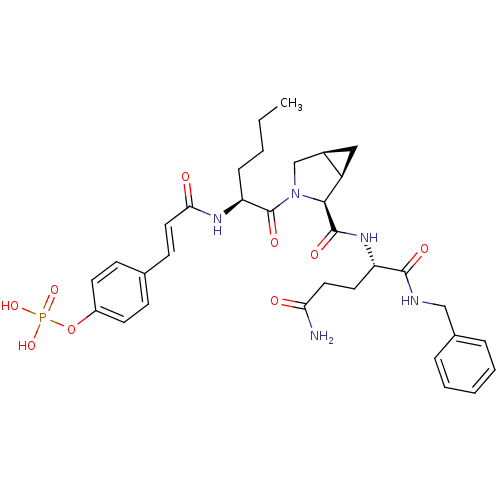
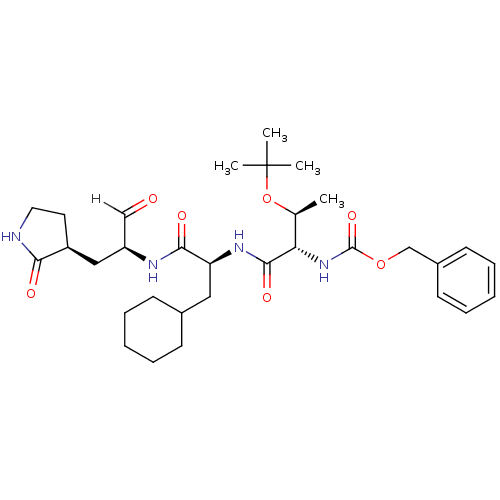
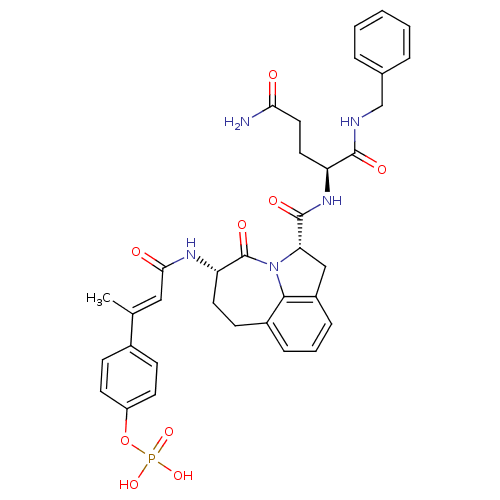
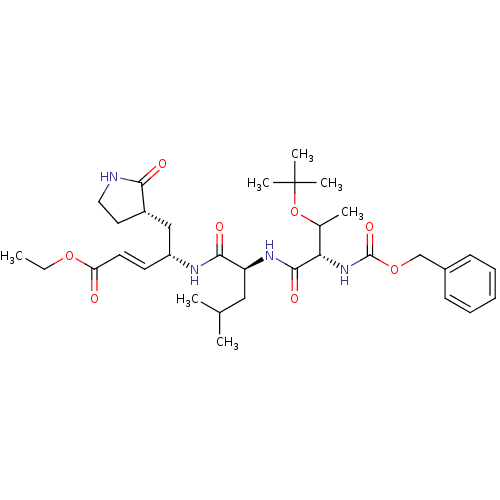
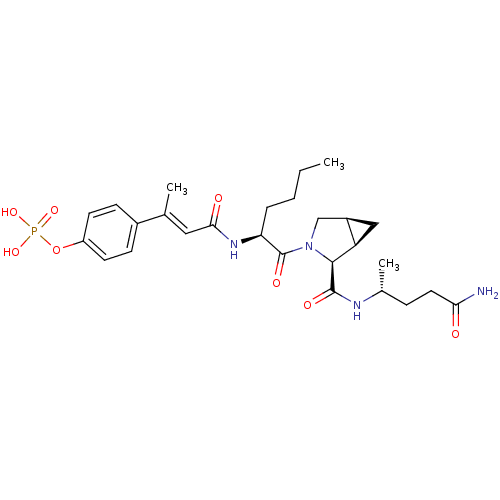
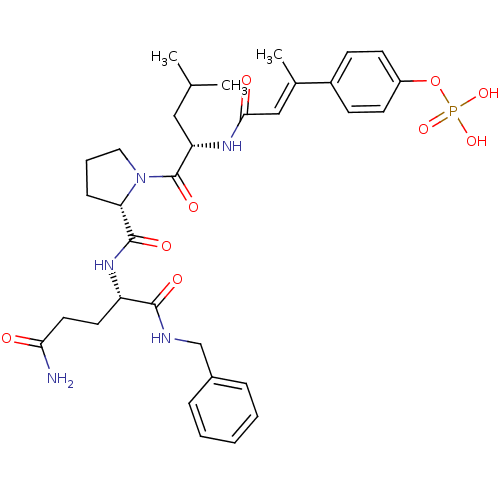
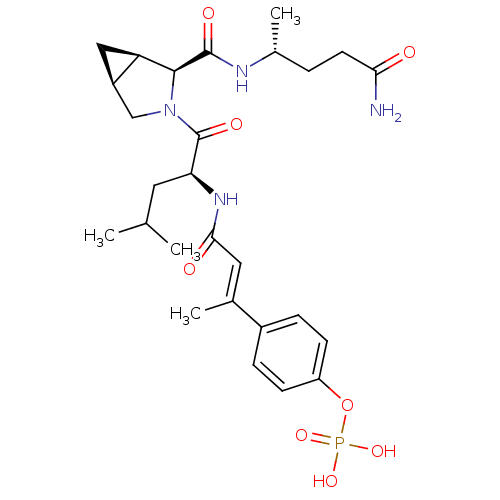
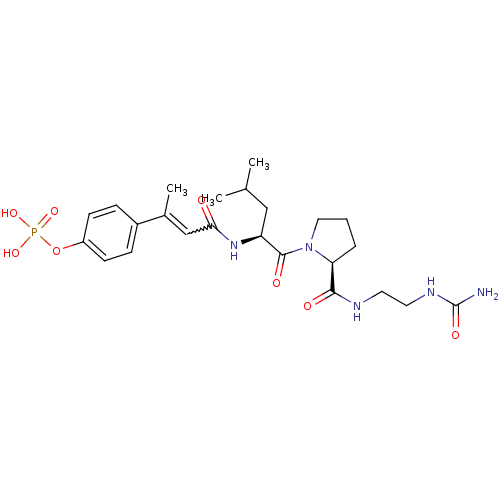
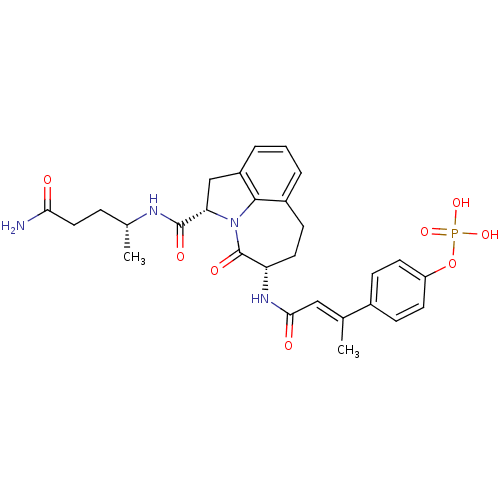
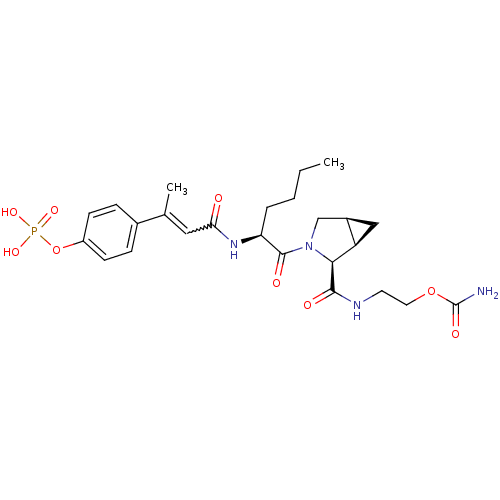
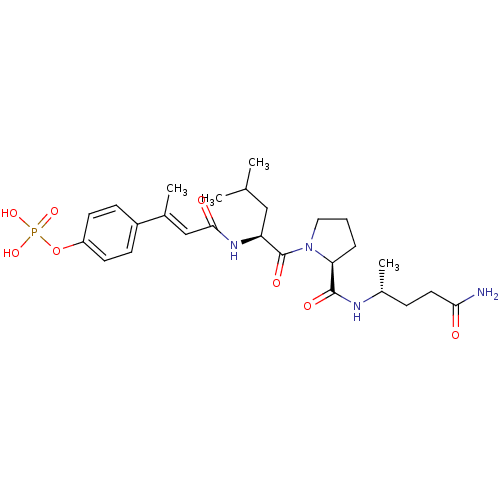
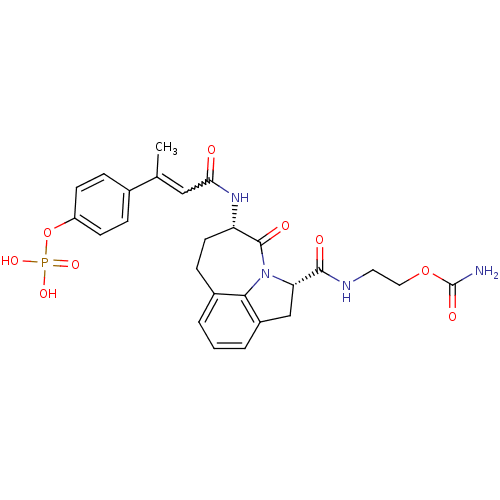

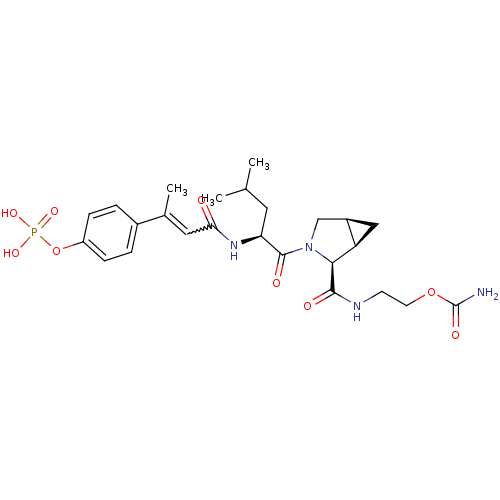
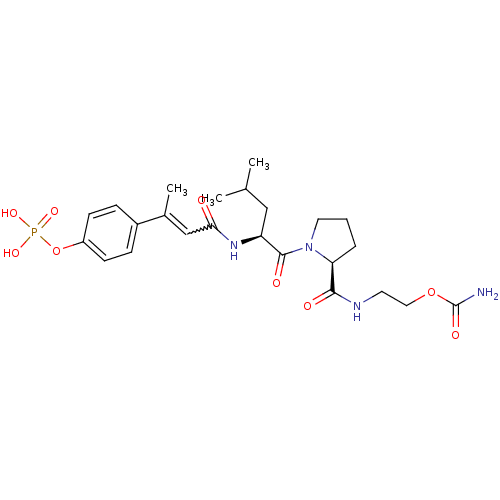
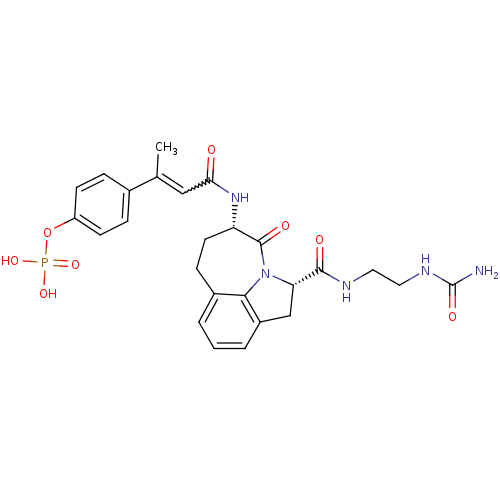
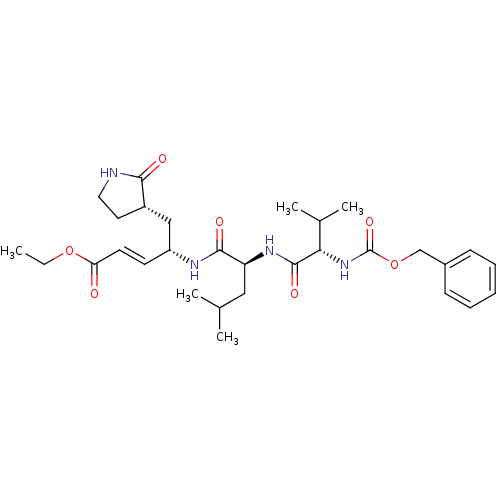
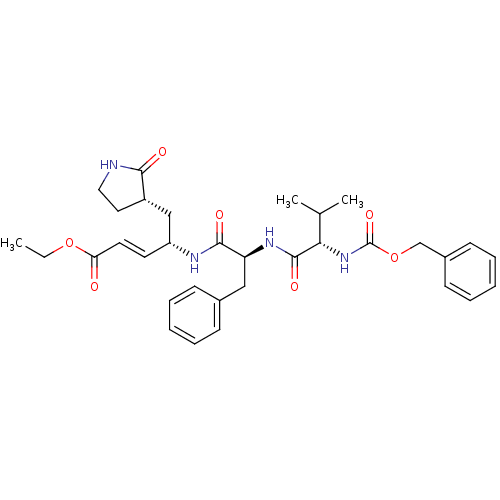

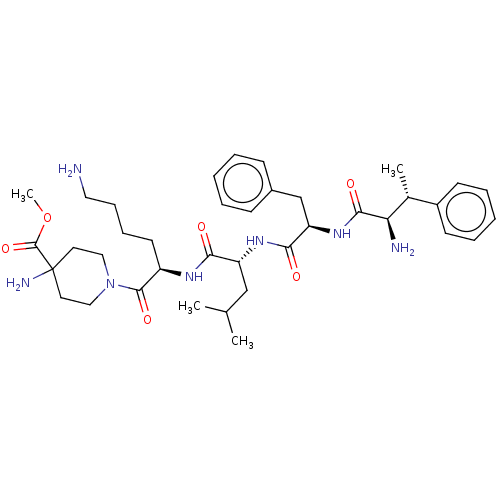
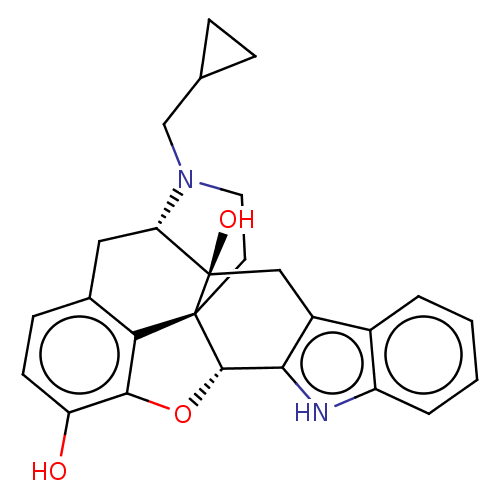
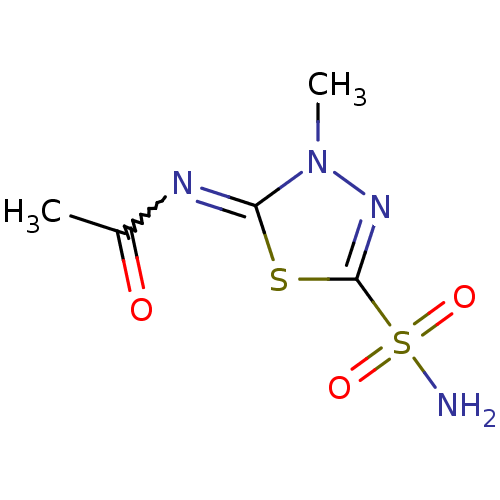
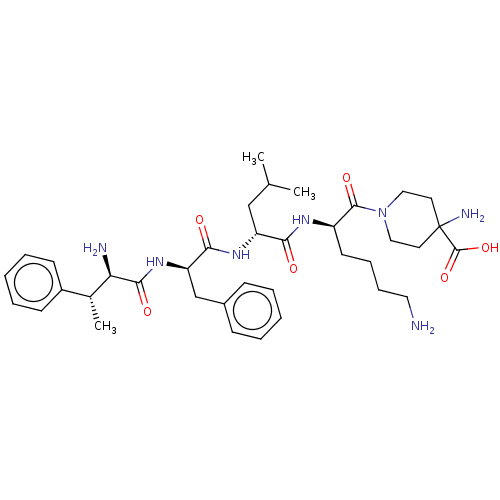
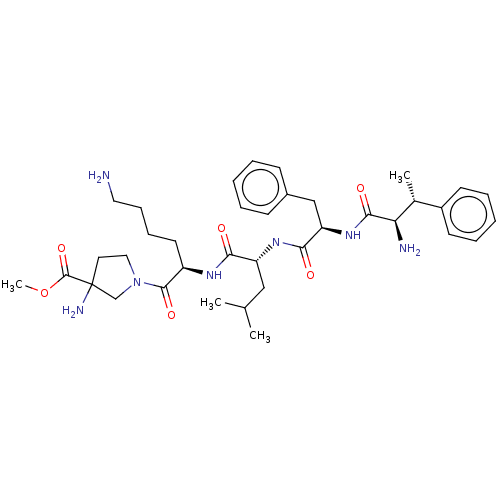
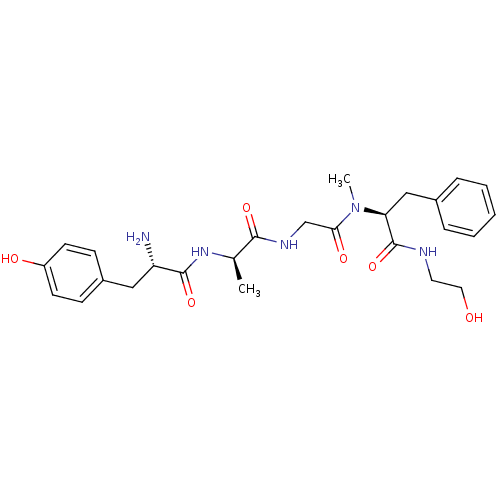
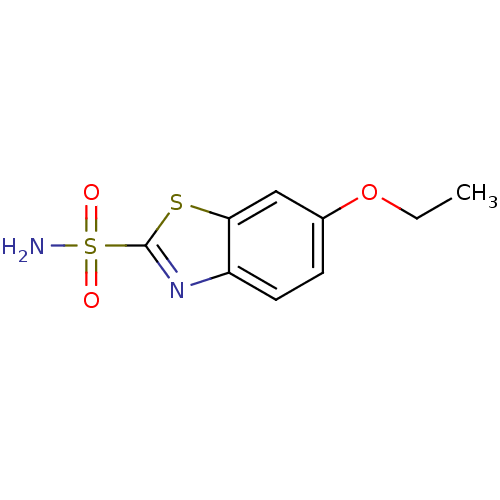
 3D Structure (crystal)
3D Structure (crystal)