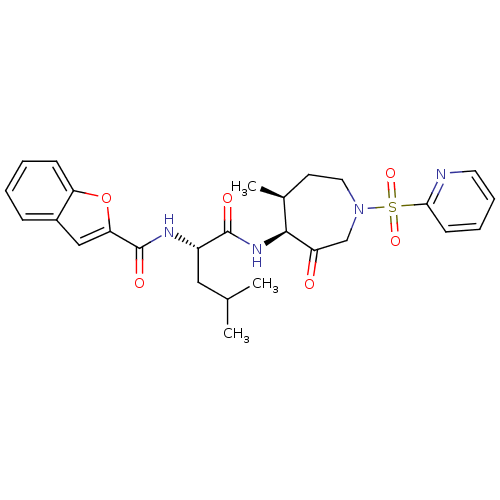
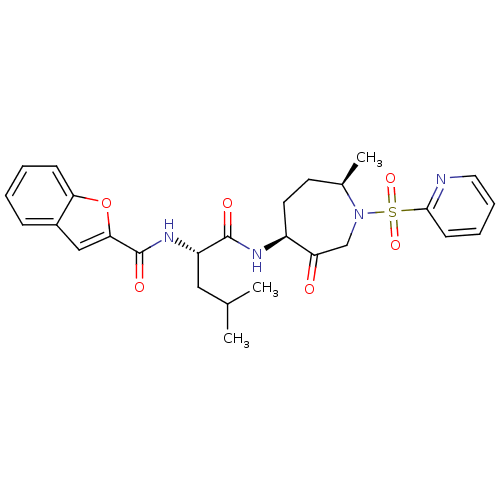
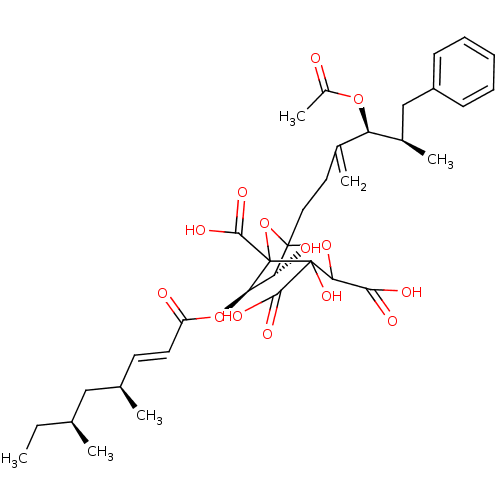
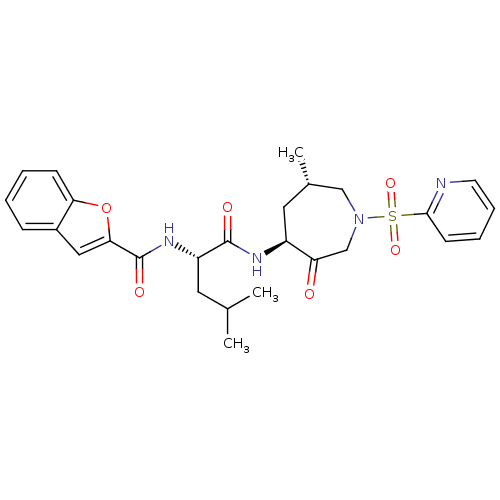
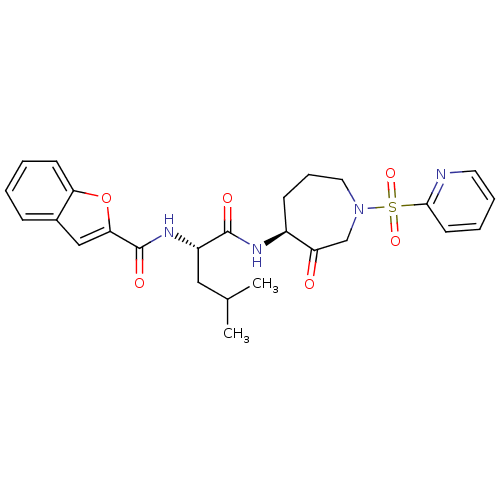
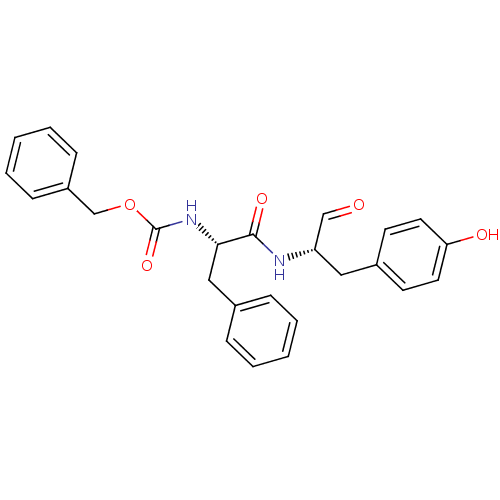
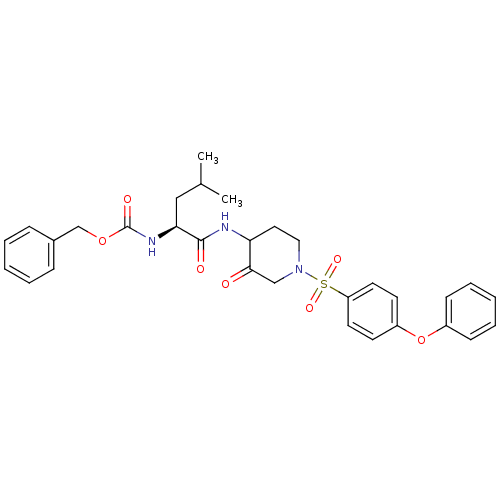
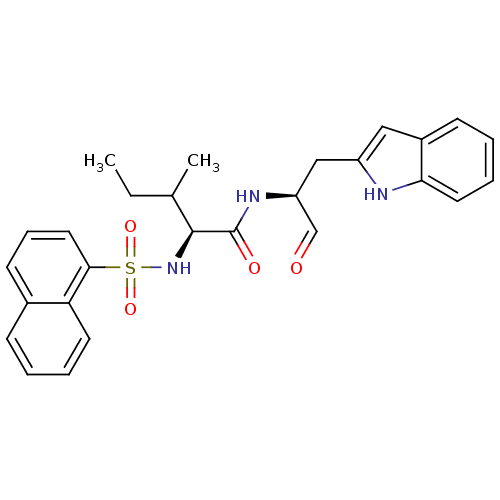
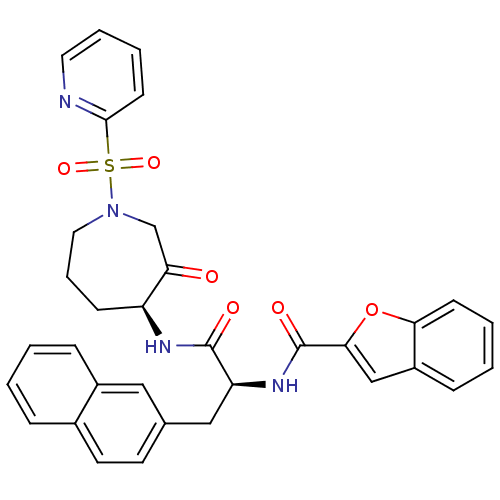
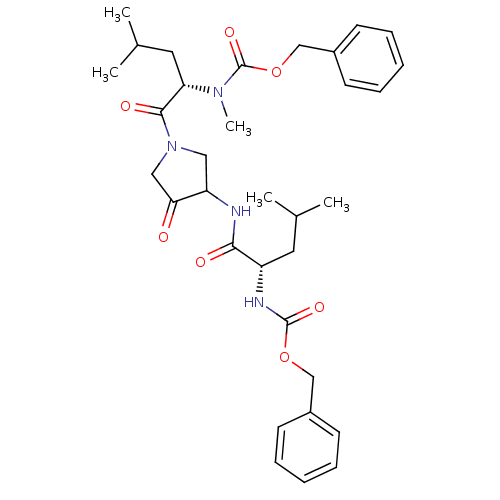
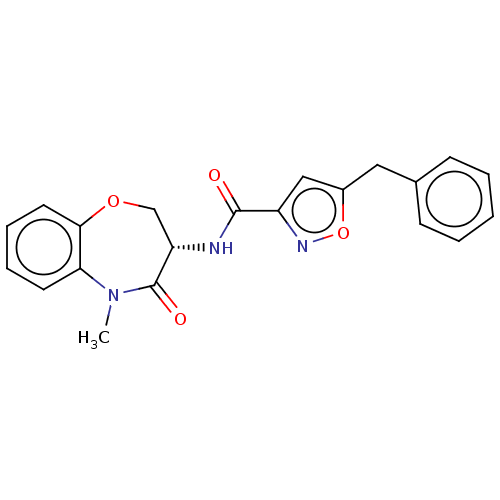
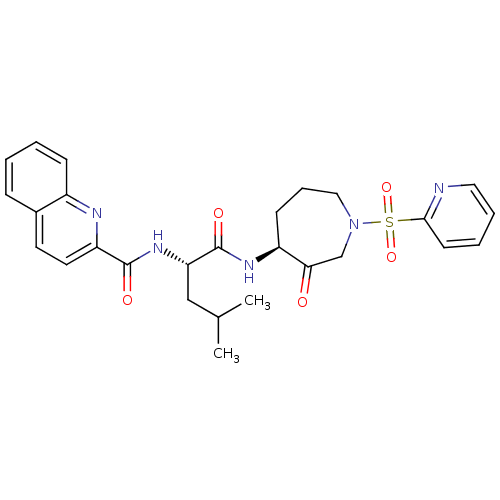
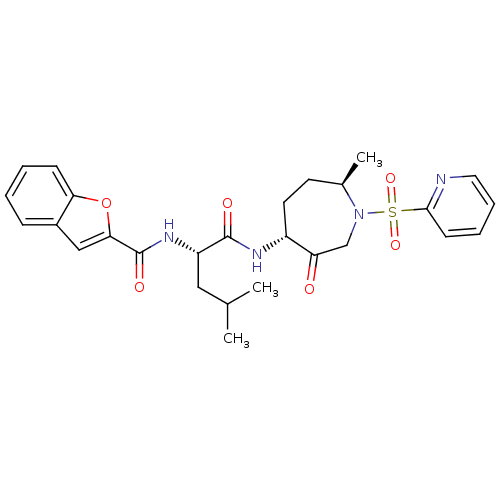
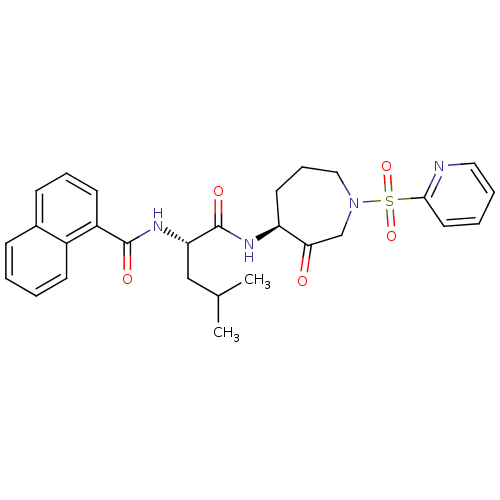
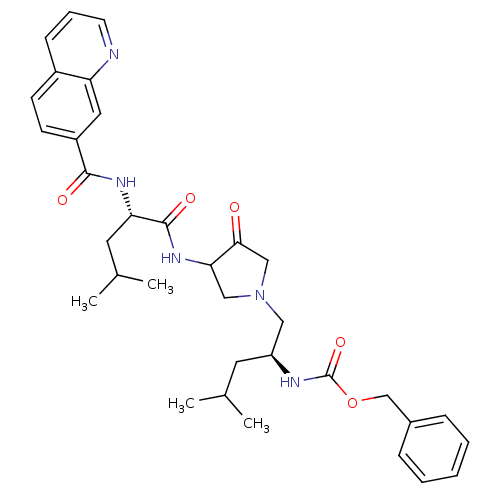
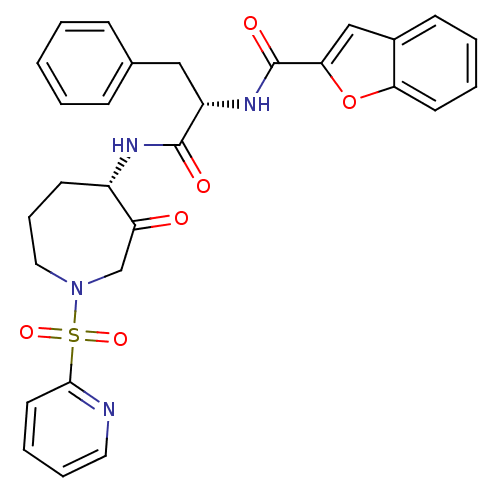
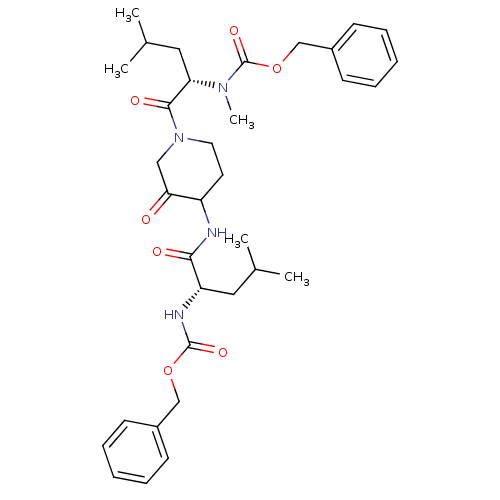
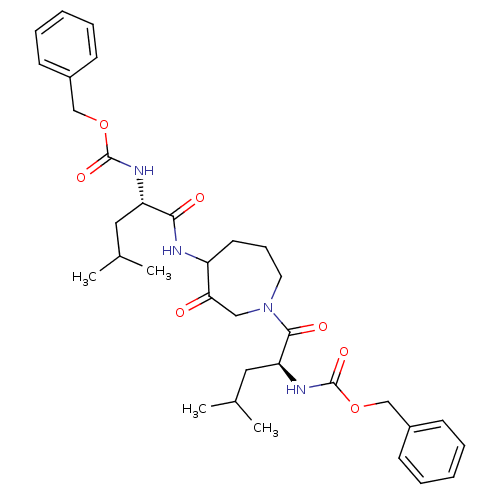
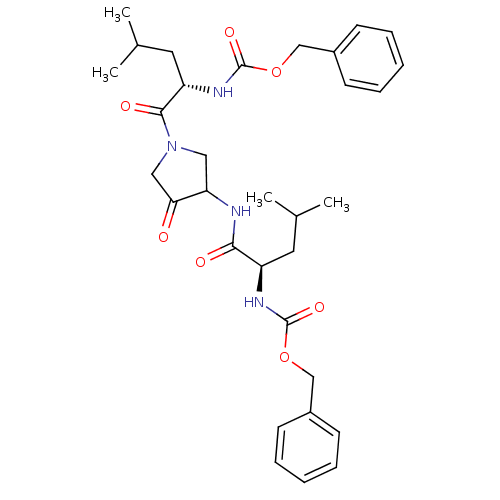
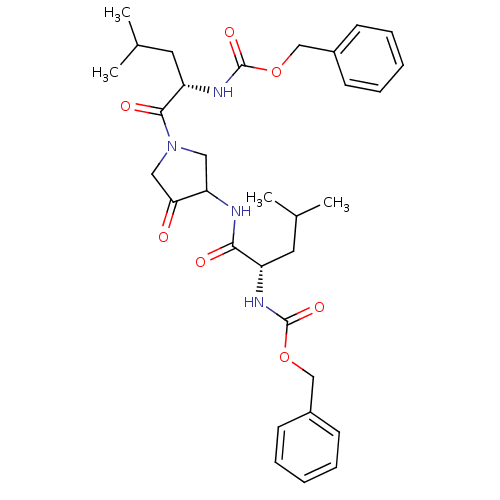
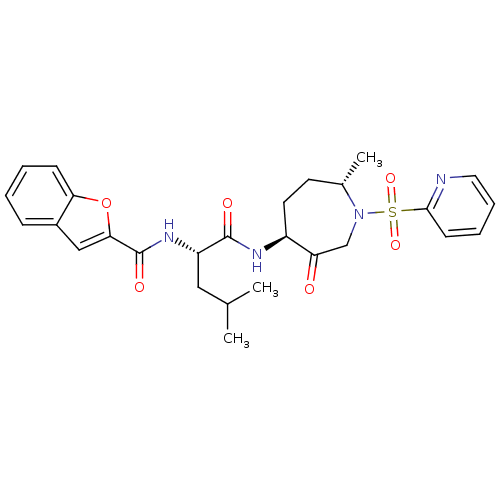
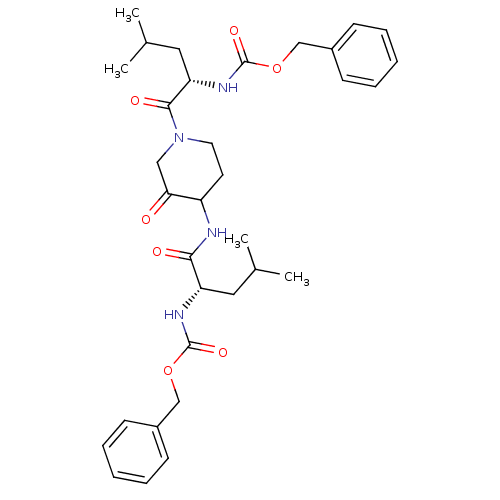
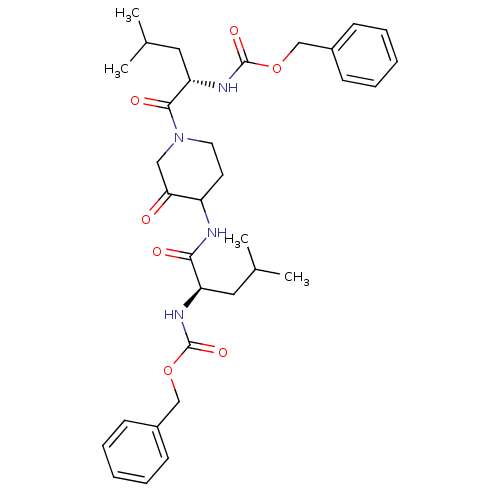
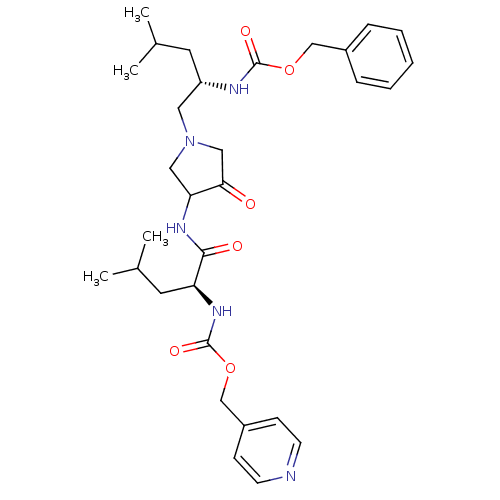
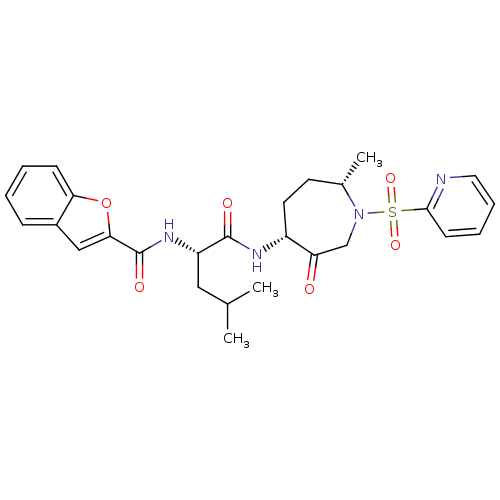
Affinity DataKi: 0.00480nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.00990nM ΔG°: -62.2kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -58.8kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0410nM ΔG°: -58.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0630nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0680nM ΔG°: -57.4kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0780nMAssay Description:Compound was tested for inhibition of squalene synthase in rat liver.More data for this Ligand-Target Pair
Affinity DataKi: 0.140nM ΔG°: -55.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nM ΔG°: -55.3kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.470nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.560nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.1kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nM ΔG°: -52.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.740nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -50.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nM ΔG°: -49.9kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.3kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nM ΔG°: -48.9kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nM ΔG°: -48.8kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -48.6kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nM ΔG°: -48.5kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nM ΔG°: -47.5kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair

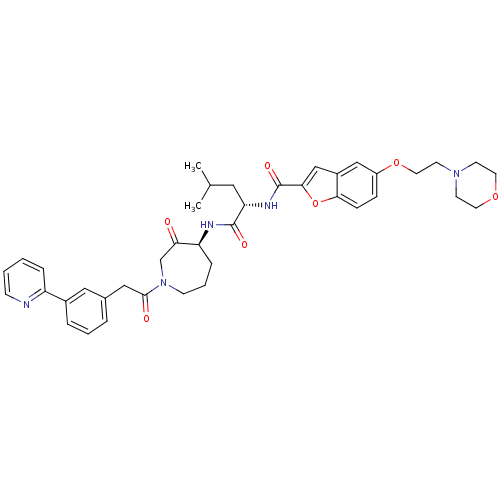
 3D Structure (crystal)
3D Structure (crystal)