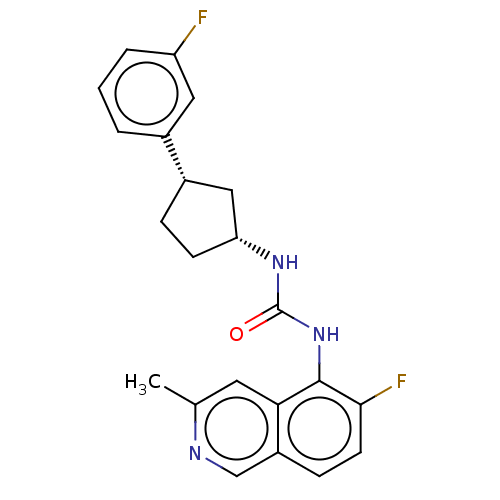
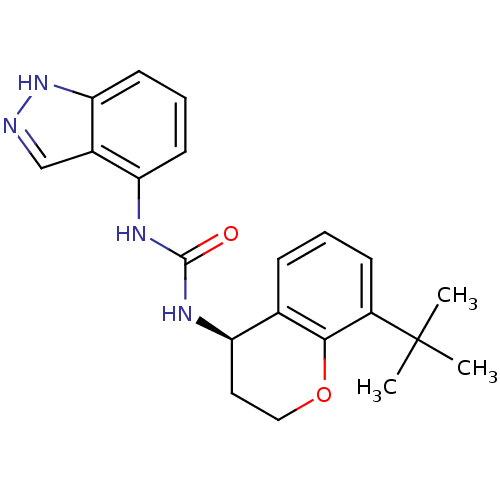
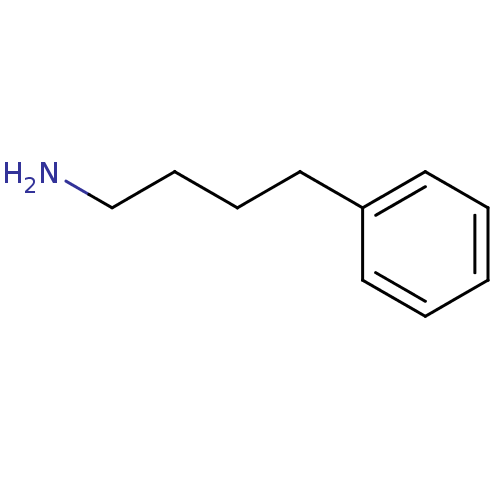TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 7nM ΔG°: -46.5kJ/mole EC50: 5nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
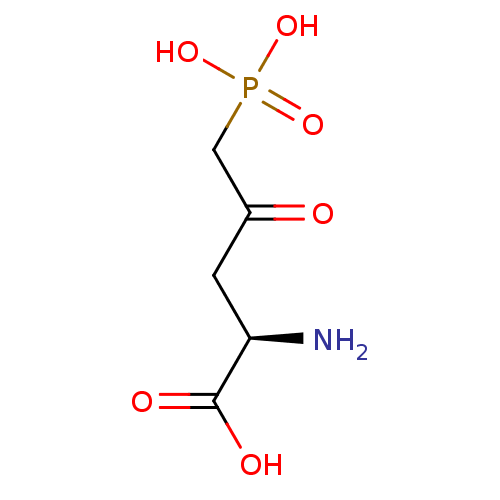
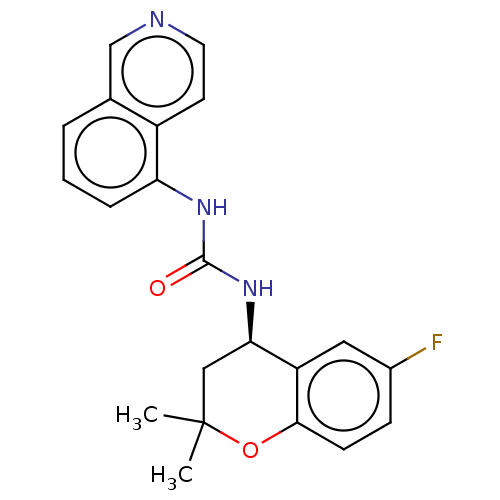
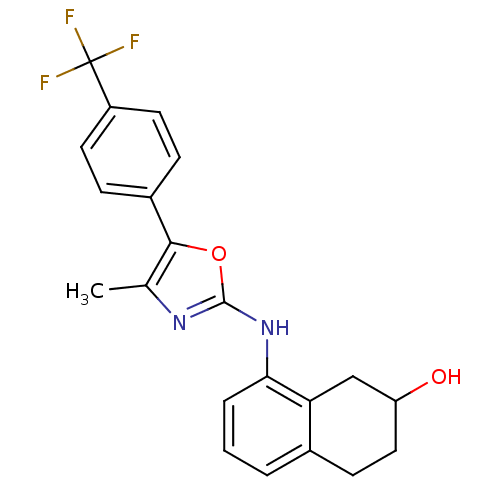
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 27nM ΔG°: -43.2kJ/mole EC50: 34nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibition of human recombinant MAOB expressed in Pichia pastoris by kinetic assayMore data for this Ligand-Target Pair
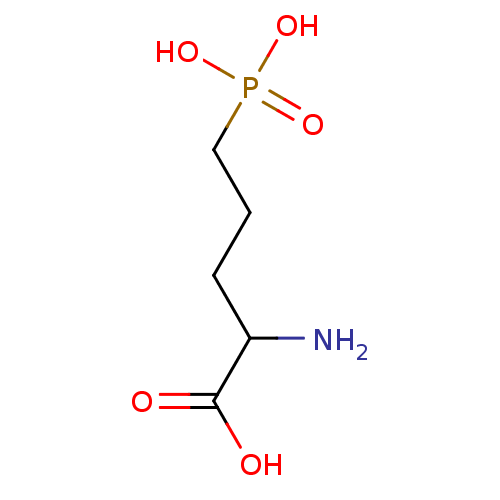
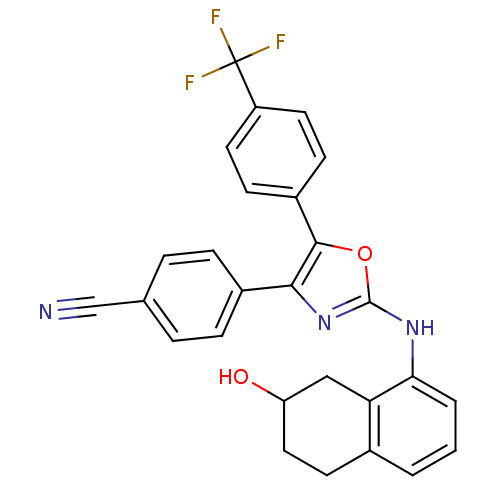
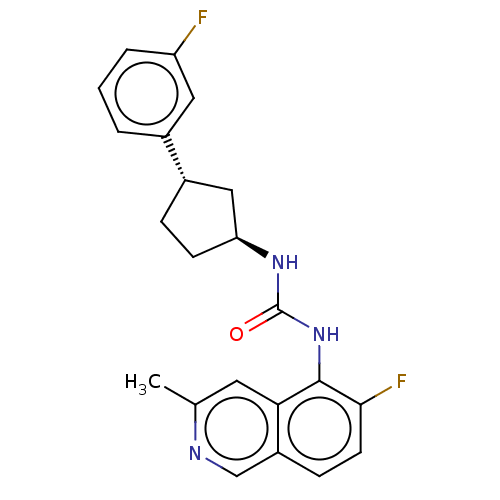
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 47nM ΔG°: -41.8kJ/mole EC50: 11nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
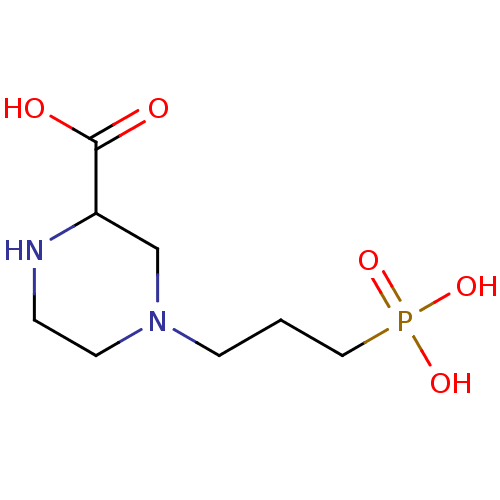
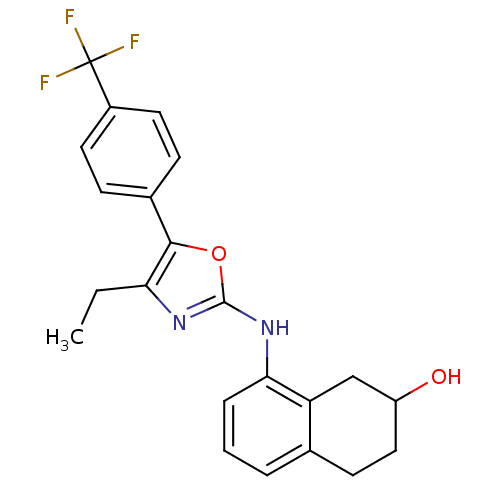
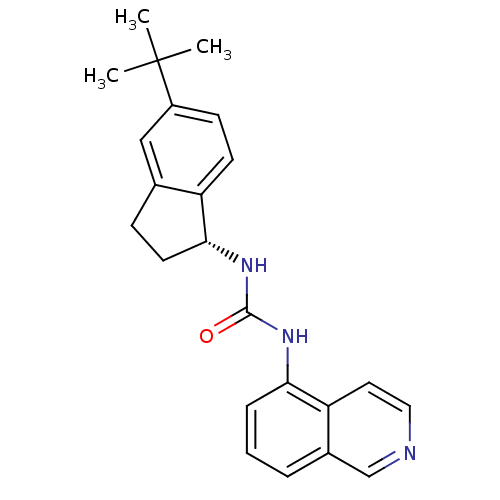
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 58nM ΔG°: -41.3kJ/mole EC50: 46nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Targetp-hydroxybenzoate hydroxylase(Pseudomonas fluorescens)
Marion Merrell Dow Research Institute
Curated by ChEMBL
Marion Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: 59nMAssay Description:Binding affinity of the compound against p-hydroxybenzoate hydroxylase (PHBH) from Pseudomonas fluorescence competing with p-hydroxy benzoic acidMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 65nM ΔG°: -41.0kJ/mole EC50: 24nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Targetp-hydroxybenzoate hydroxylase(Pseudomonas fluorescens)
Marion Merrell Dow Research Institute
Curated by ChEMBL
Marion Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: 69nMAssay Description:Binding affinity of the compound against p-hydroxybenzoate hydroxylase (PHBH) from Pseudomonas fluorescence competing with NADPHMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 71nM ΔG°: -40.8kJ/mole EC50: 74nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 112nM ΔG°: -39.7kJ/mole EC50: 34nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 589nM ΔG°: -35.6kJ/mole EC50: 129nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 603nM ΔG°: -35.5kJ/mole EC50: 95nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of human recombinant MAOA expressed in Pichia pastoris by kinetic assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 1.29E+3nM ΔG°: -33.6kJ/mole EC50: 282nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 1.59E+3nM ΔG°: -33.1kJ/mole EC50: 132nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Affinity DataKi: 1.61E+3nMAssay Description:Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.64E+3nMAssay Description:Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysisMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: >6.31E+3nM ΔG°: >-29.7kJ/mole EC50: 1.48E+3nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 2.00E+4nM ΔG°: -26.8kJ/mole EC50: 29nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+4nMAssay Description:Inhibition of human MAOAMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 0.700nMpH: 5.5Assay Description:Blockade of pH 5.5-induced activation of TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.30nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at mineralocorticoid receptor ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2.30nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Binding affinity towards Nicotinic acetylcholine receptor by the displacement of [3H]-nicotine from rat cortical membranesMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Blockade of N-arachidonoyl-dopamine-induced activation of TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Blockade of human TRPV1 receptor assessed as inhibition of capsaicin-induced calcium fluxMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at glucocorticoid receptor ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by luc...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.30nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.5nMAssay Description:Antagonist activity at human TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.5nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of rat membrane MAOBMore data for this Ligand-Target Pair

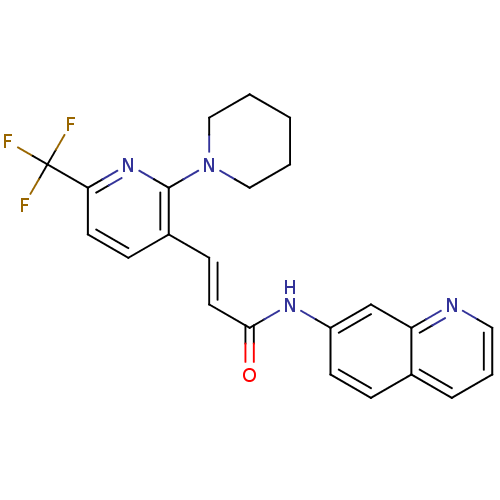
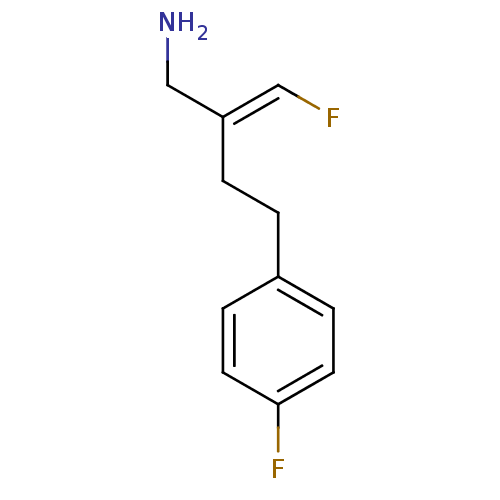
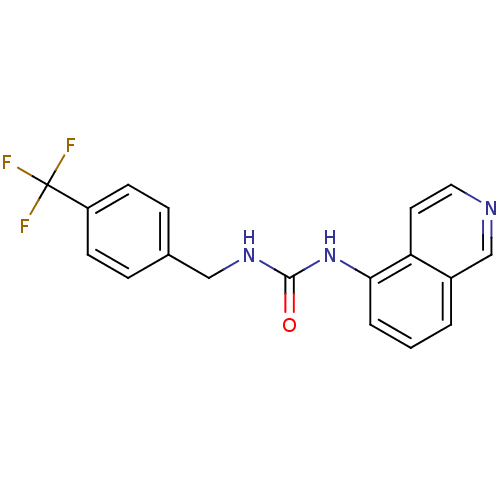

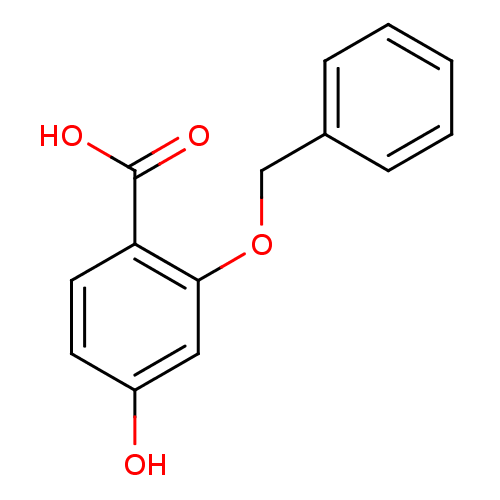
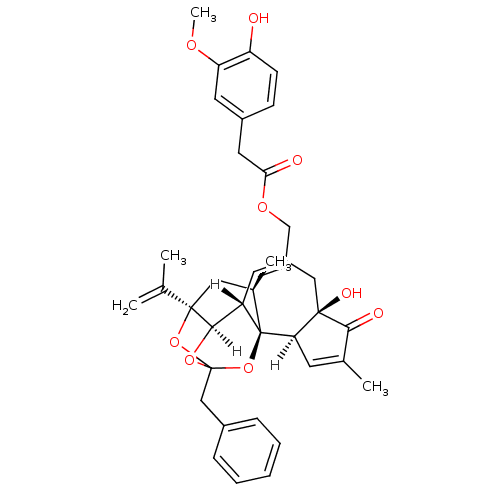
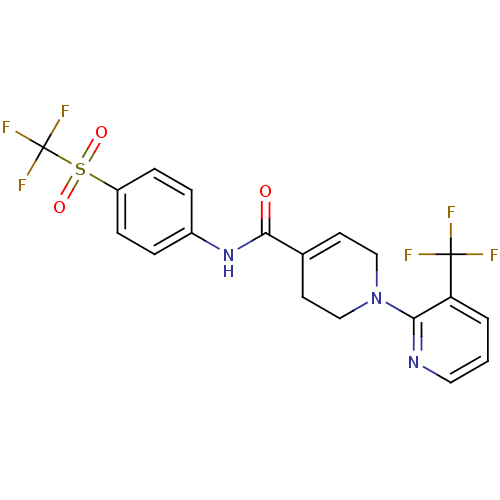


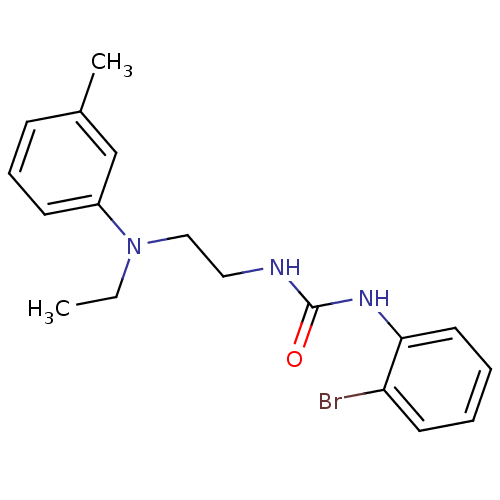
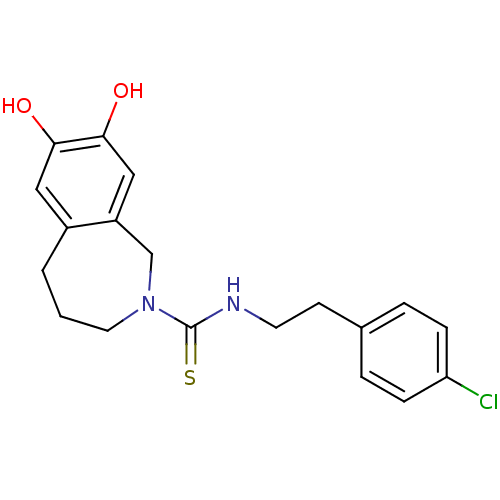
 3D Structure (crystal)
3D Structure (crystal)