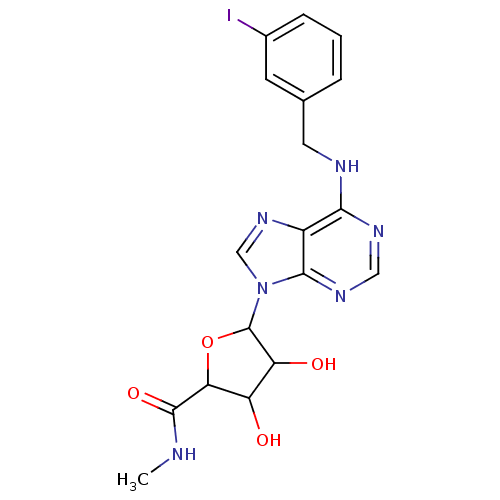
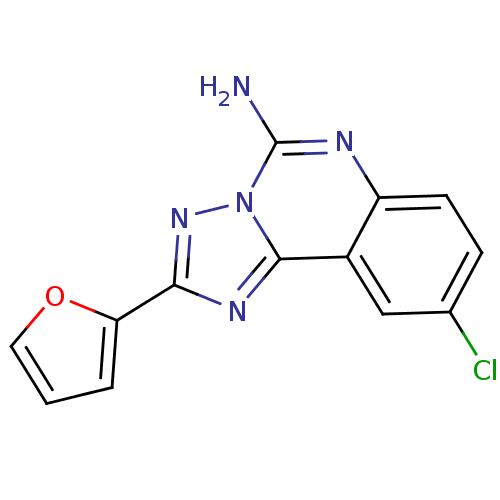
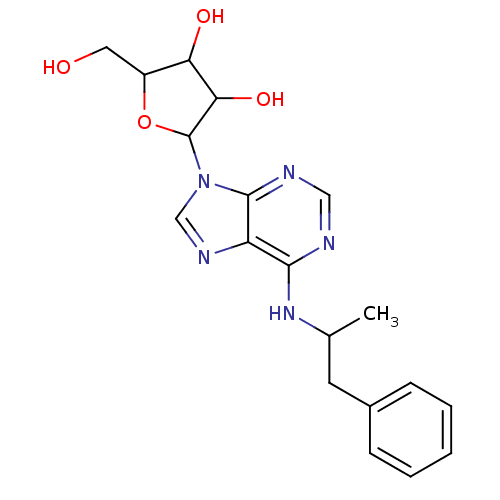
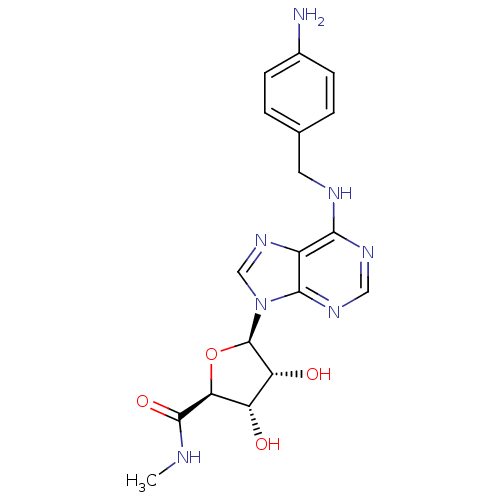
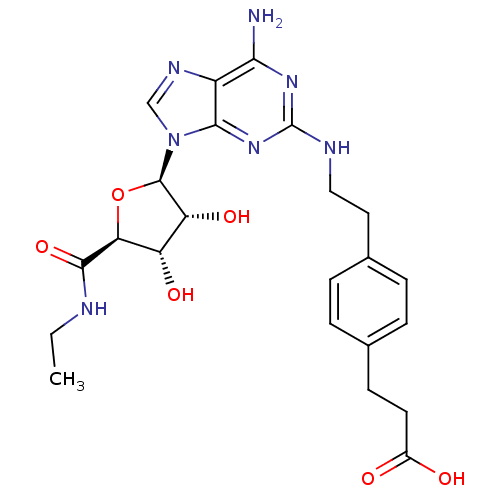
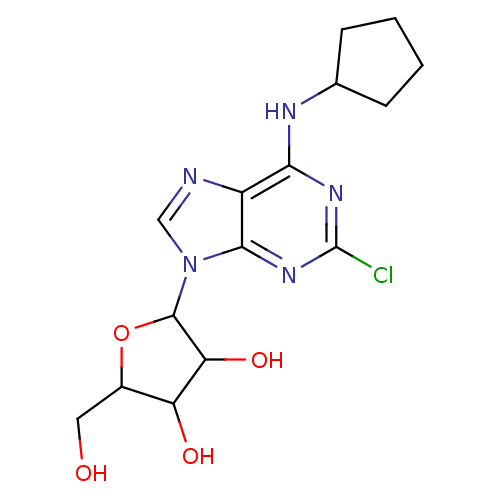
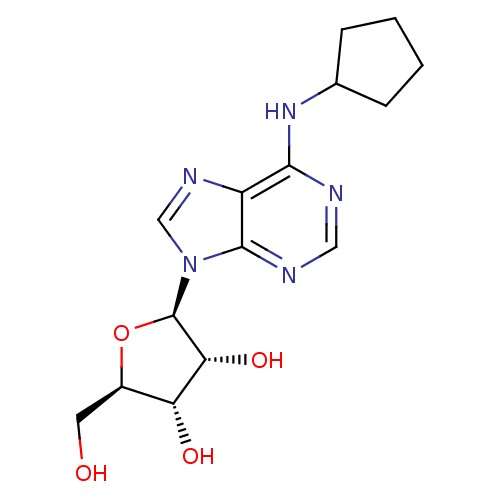
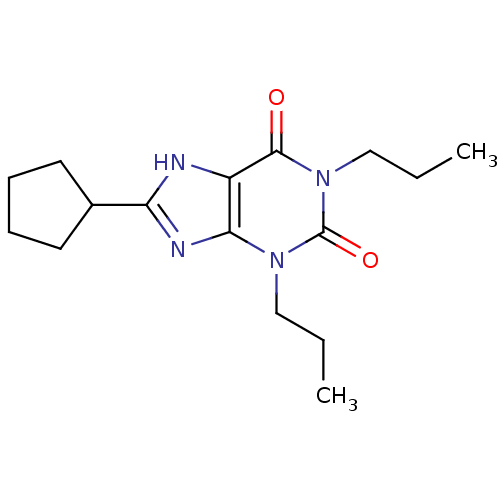
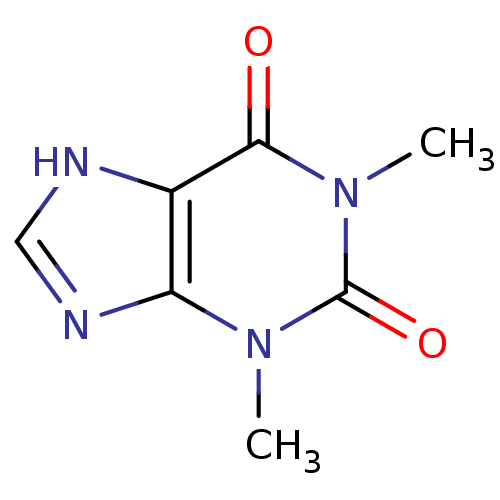
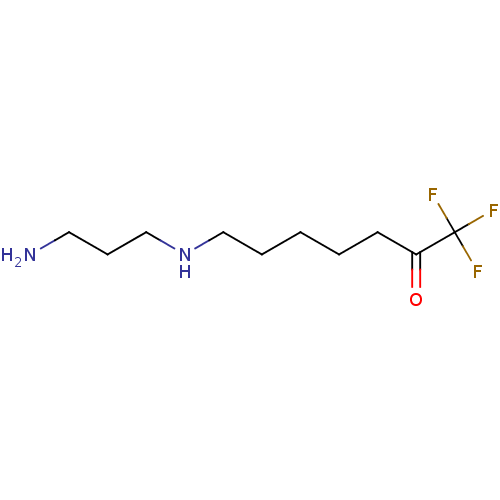
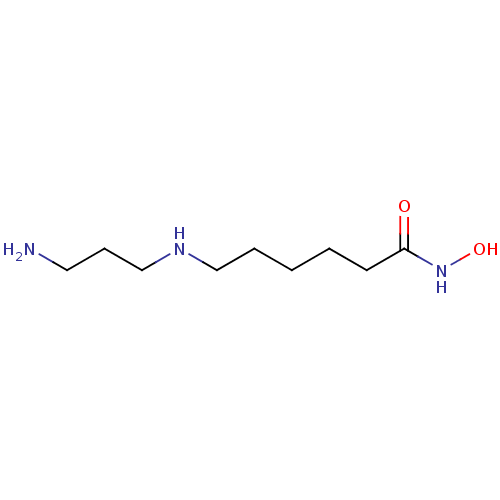
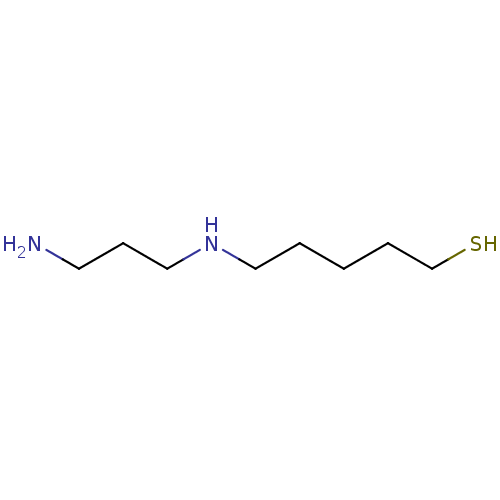
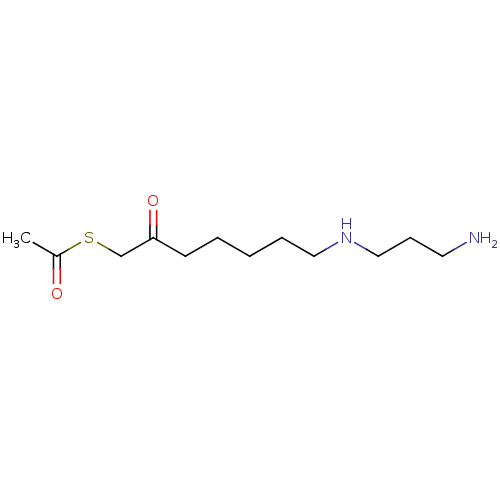
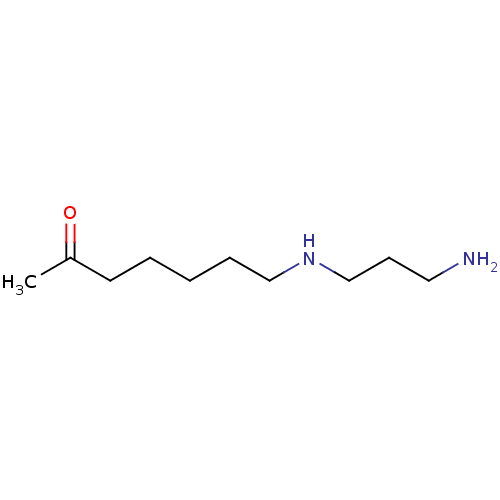
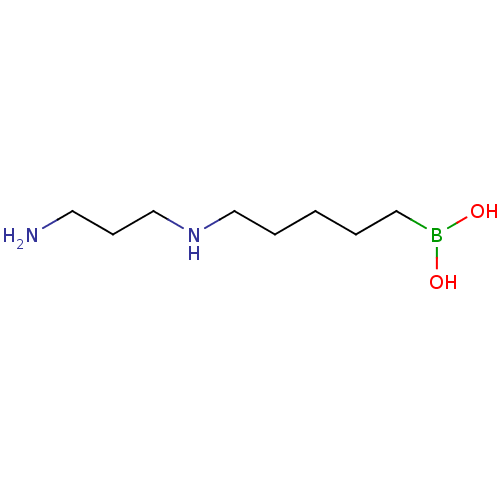
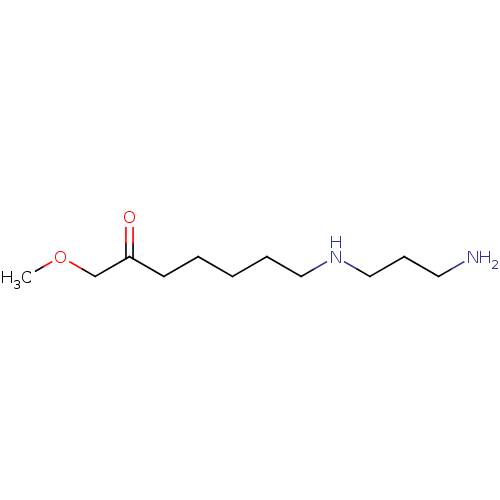
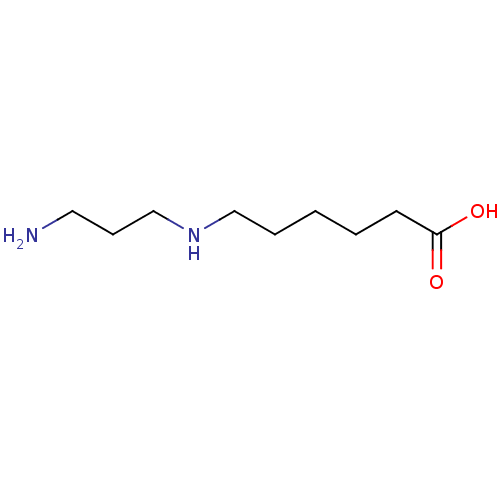
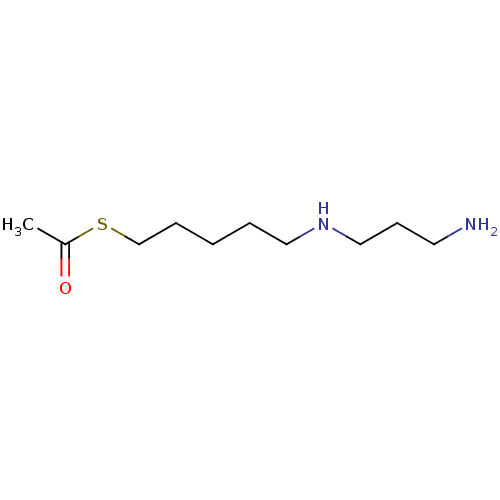
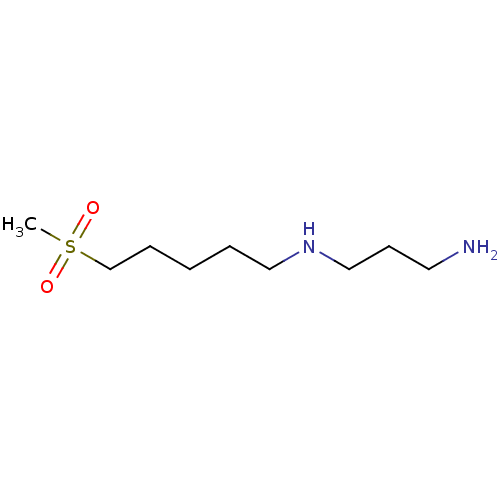
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
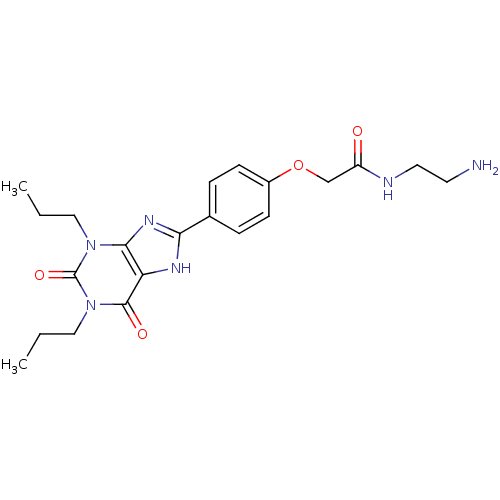
Affinity DataIC50: 270nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.60E+5nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 2.30E+5nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.80E+5nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.80E+6nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.90E+6nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair
TargetAcetylpolyamine amidohydrolase(Mycoplana ramosa (Gram-negative bacterium))
University Of Pennsylvania
Curated by ChEMBL
University Of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assayMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)