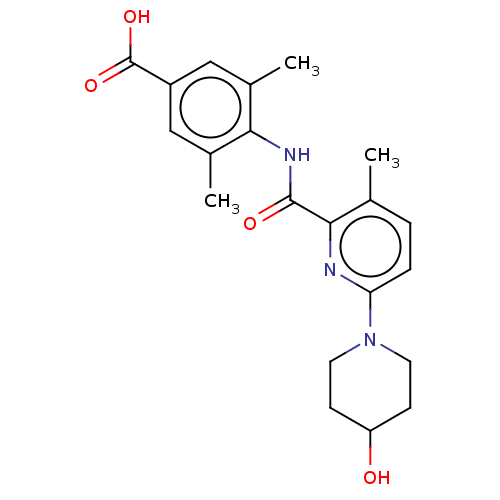
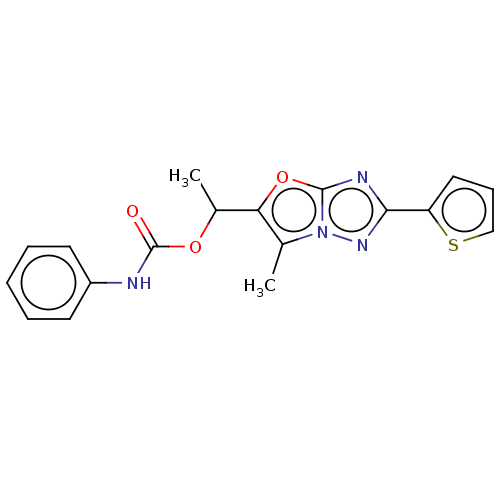
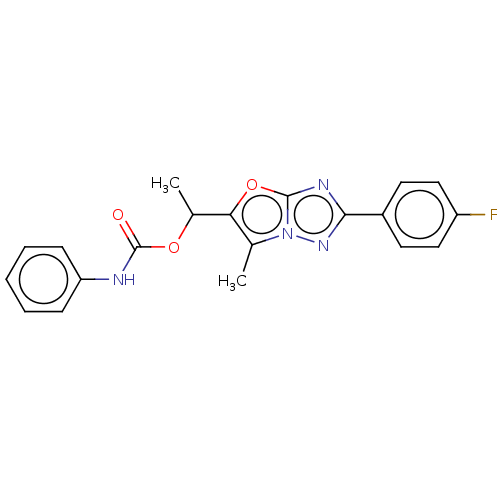
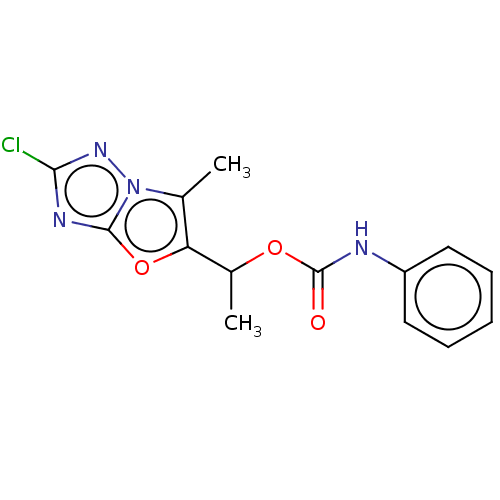
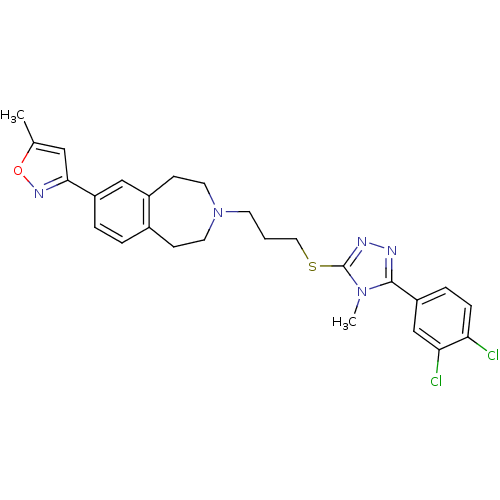
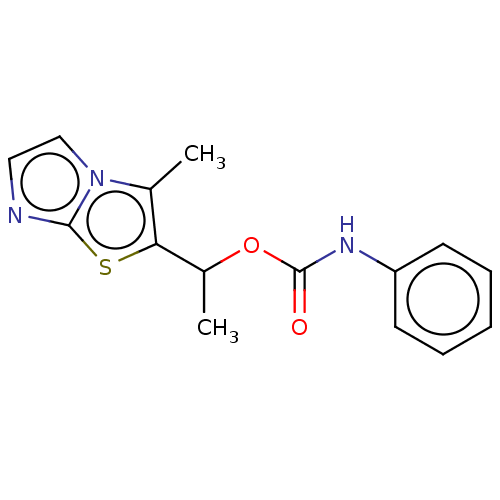
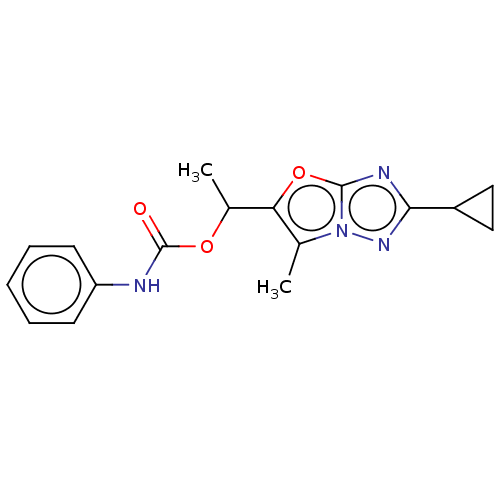
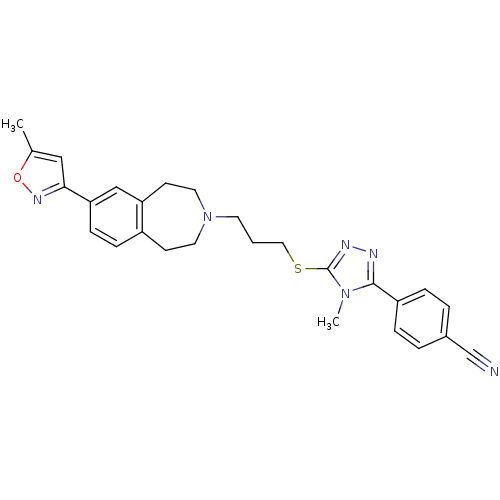
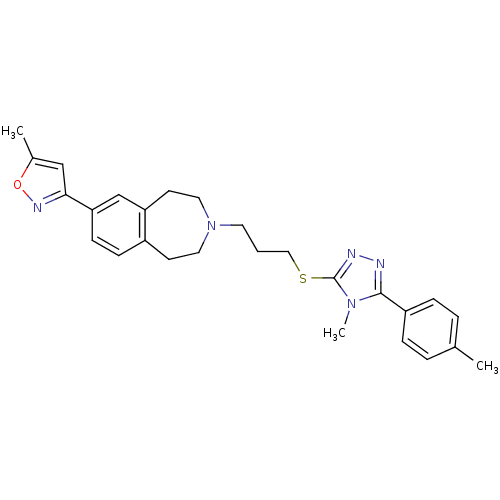
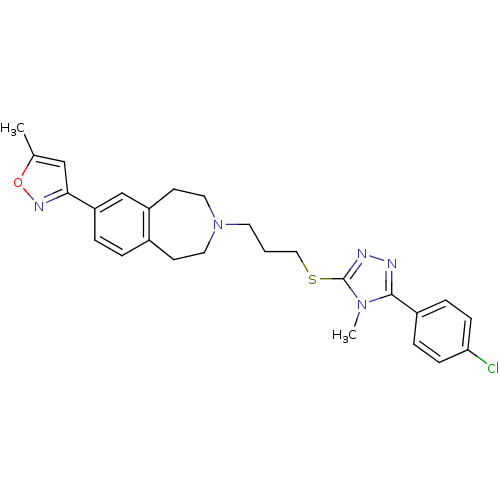
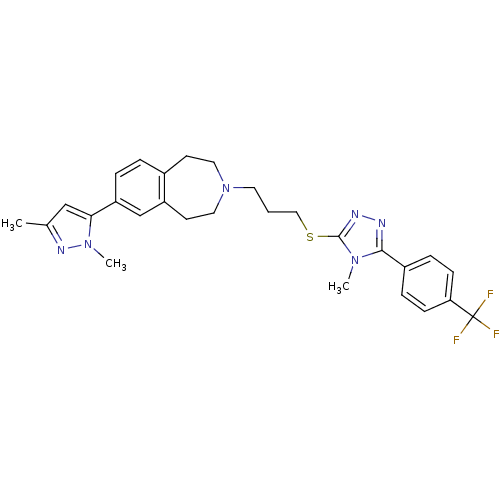
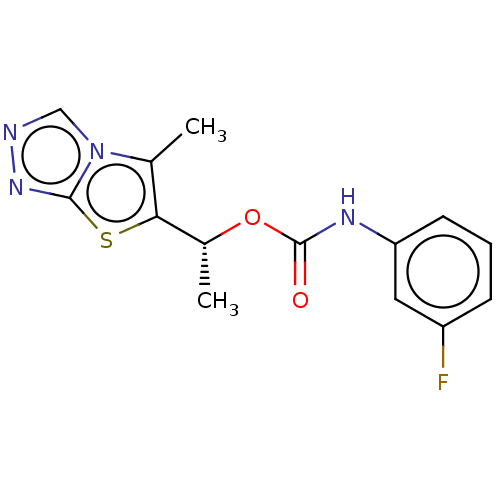
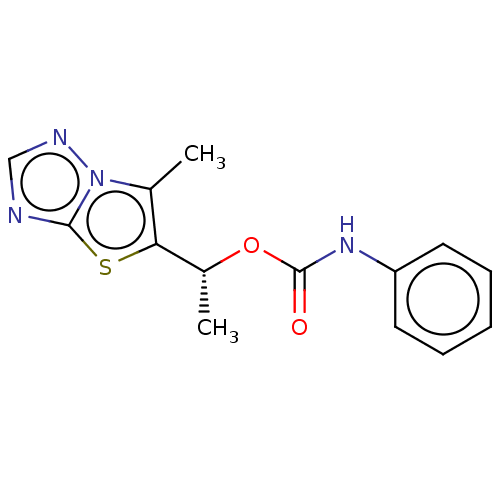
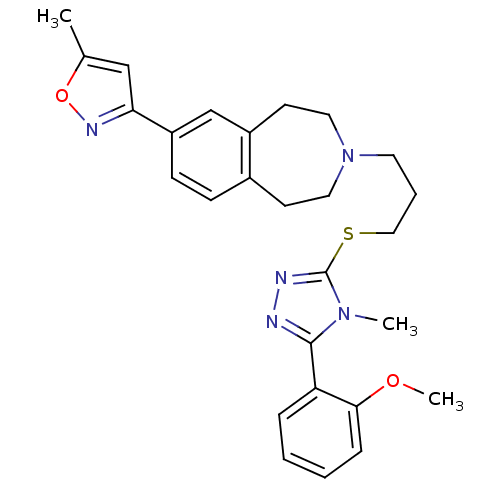
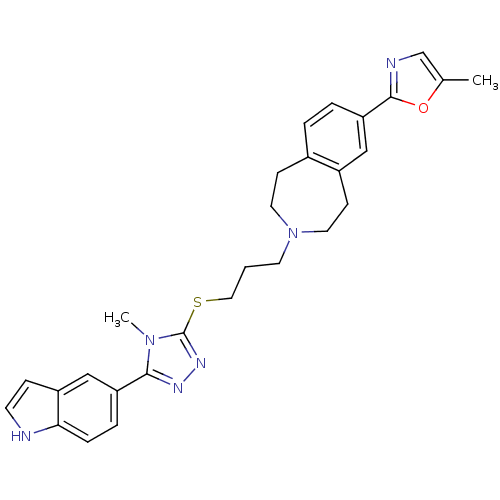
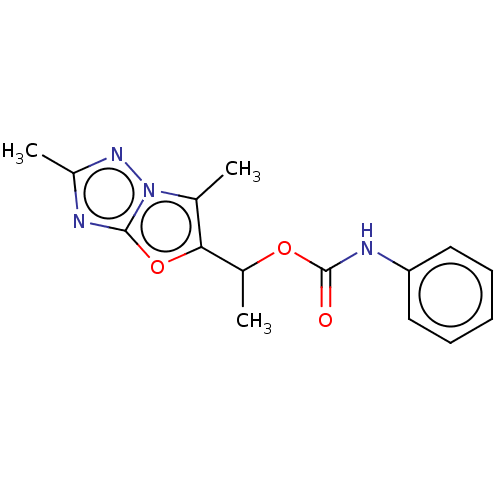
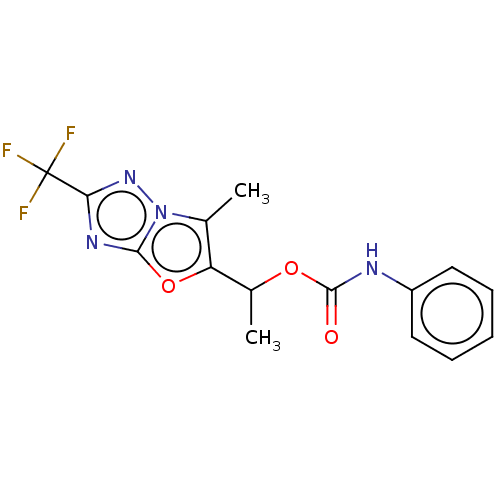
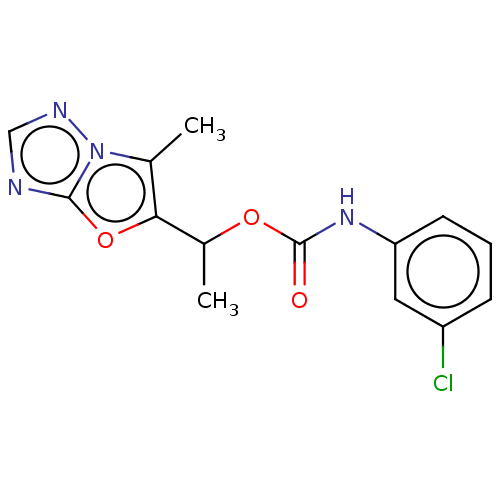
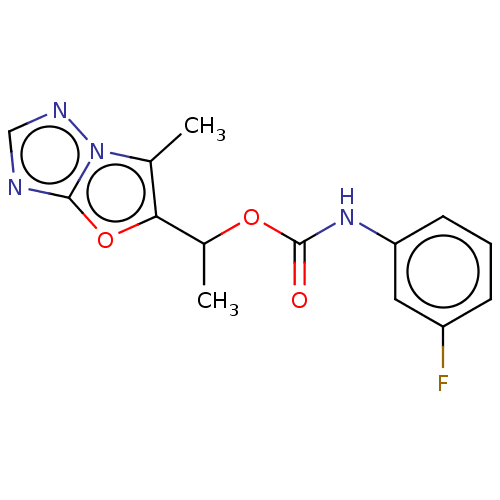
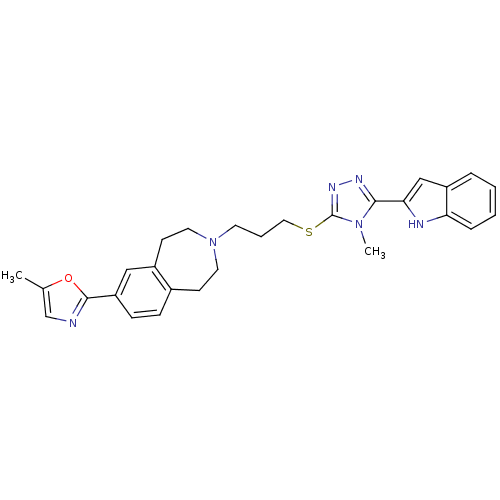
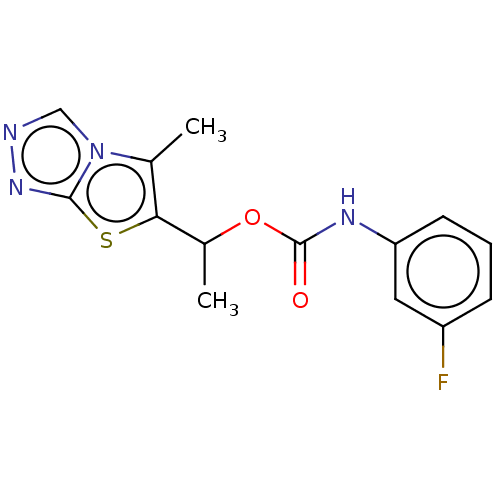
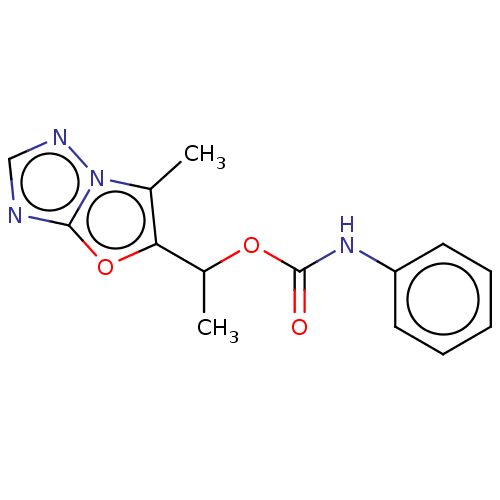
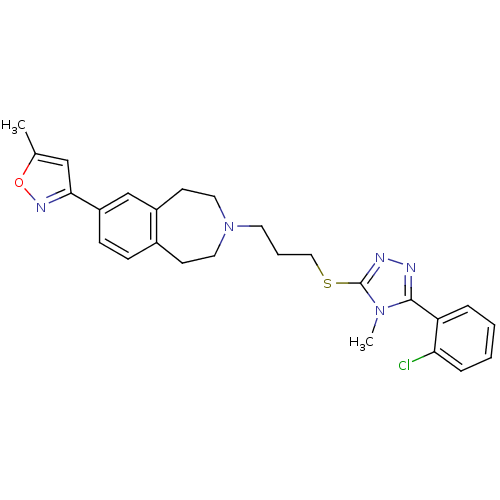
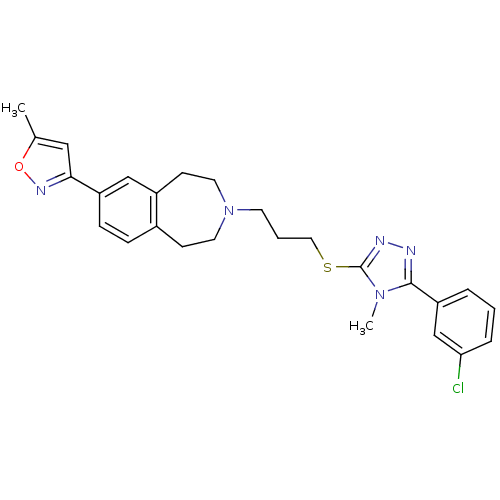
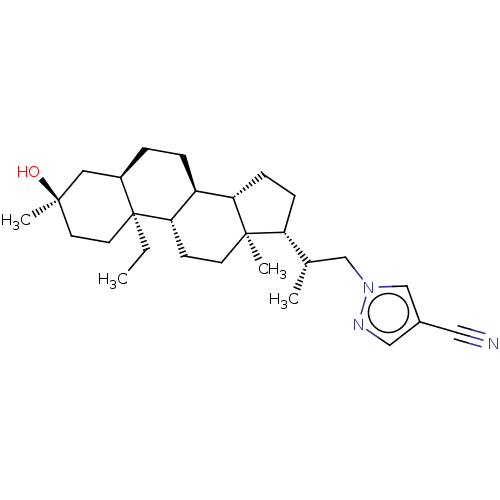
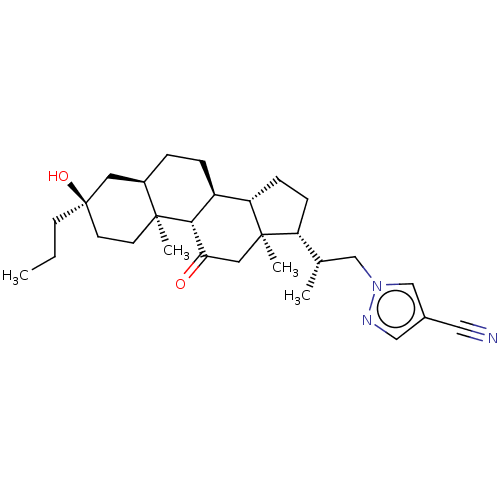
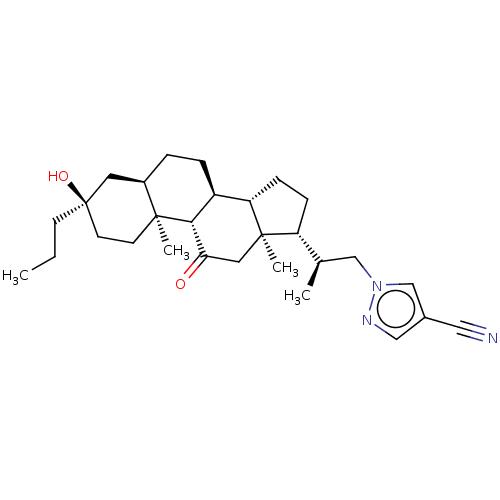
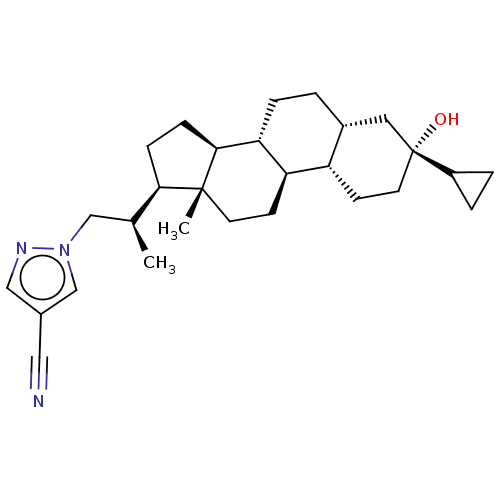
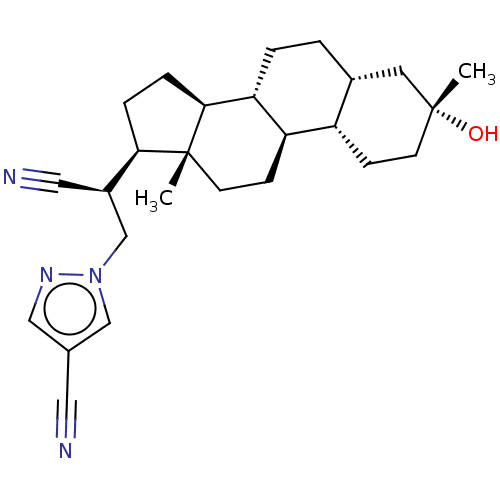
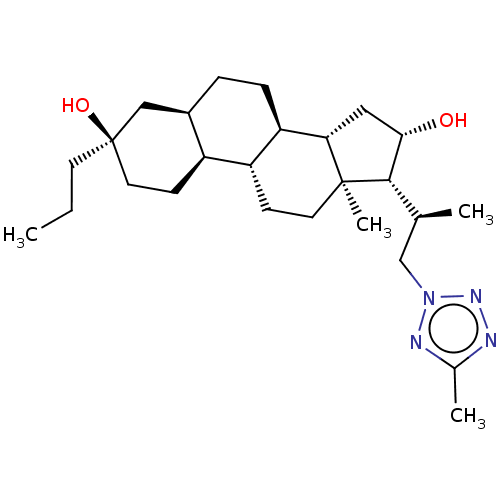
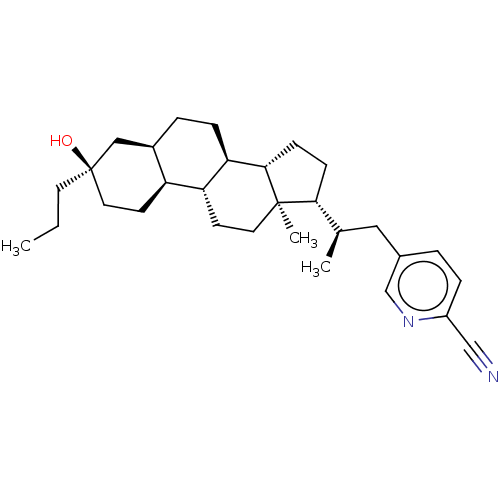
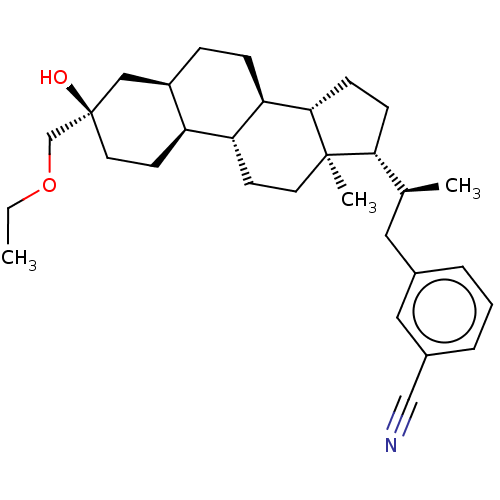
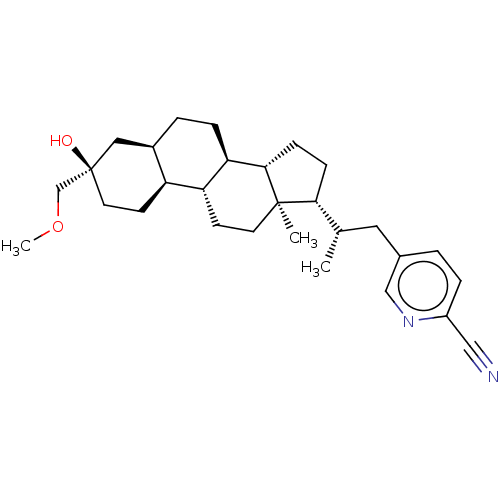
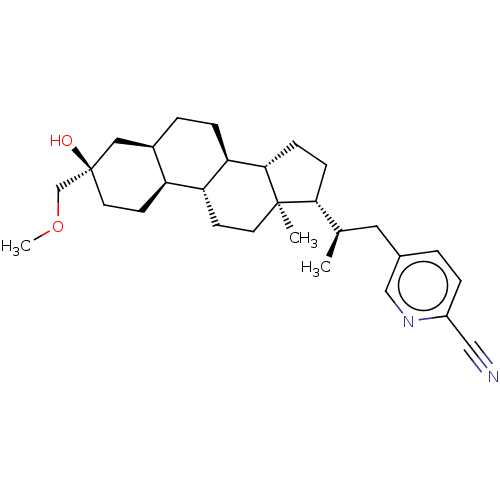
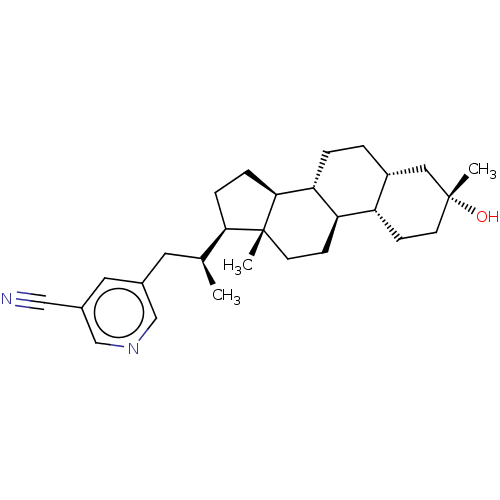
Affinity DataKi: 76nMpH: 7.4Assay Description:hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac...More data for this Ligand-Target Pair
Affinity DataKi: >1.40E+4nMpH: 7.4Assay Description:hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac...More data for this Ligand-Target Pair
Affinity DataKi: >1.75E+4nMpH: 7.4Assay Description:hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac...More data for this Ligand-Target Pair
Affinity DataKi: >1.89E+4nMpH: 7.4Assay Description:hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 19.9nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 25.1nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 25.1nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 31.6nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Antagonist activity at mGluR5 in mouse astrocytes assessed as inhibition of L-quisqualate induced calcium release by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Antagonist activity at mGluR5 in mouse astrocytes assessed as inhibition of L-quisqualate induced calcium release by FLIPR assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 39.8nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 39.8nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 39.8nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 50.1nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Medicines Research Centre
Curated by ChEMBL
Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilizationMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 63.1nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 63.1nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 79.4nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair