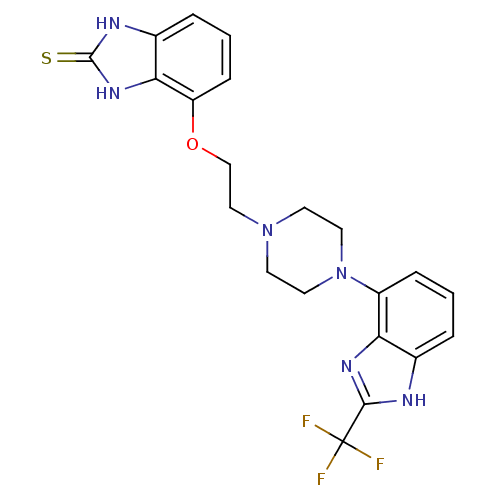
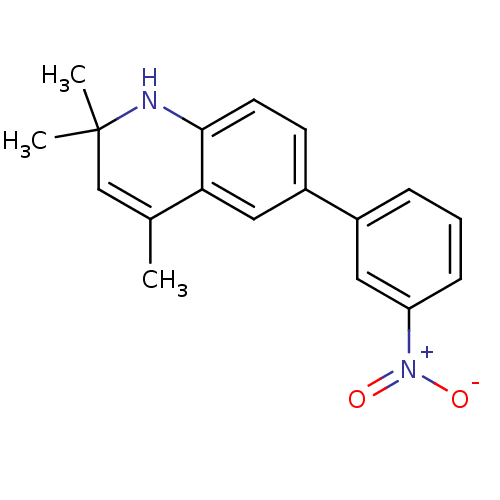
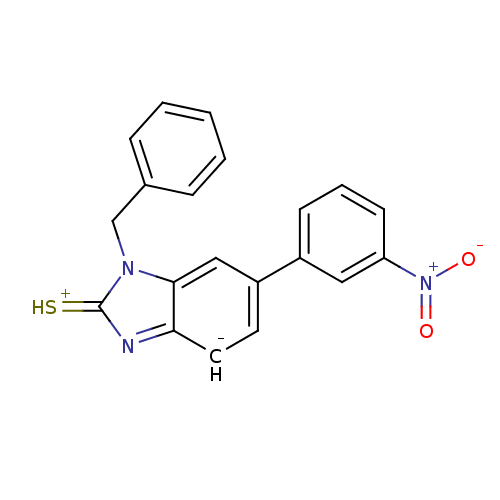
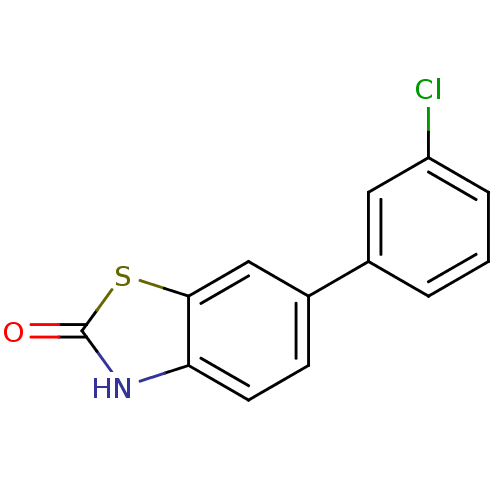
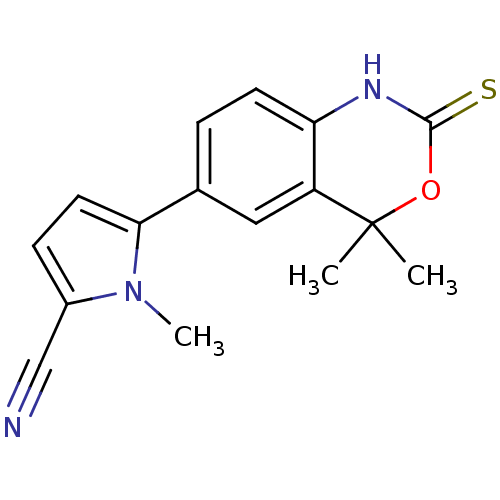
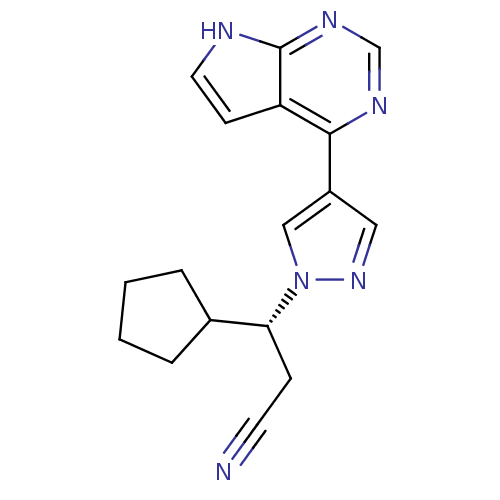
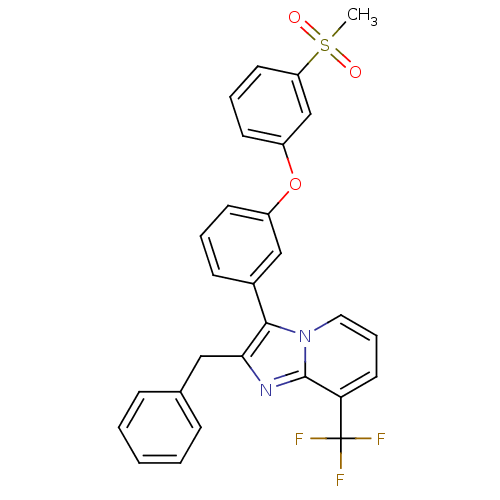
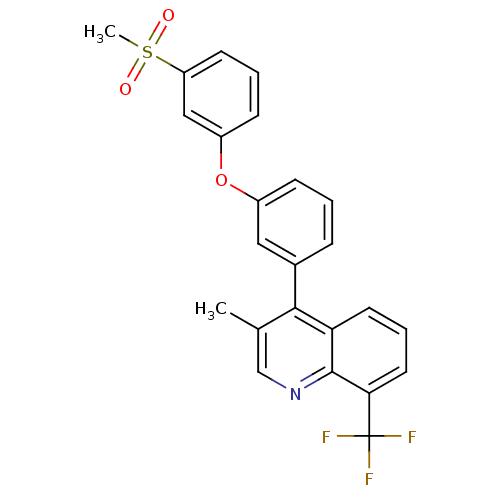
Affinity DataKi: 0.550nMAssay Description:Binding affinity to human 5HT1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Binding affinity to human 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity to human 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Binding affinity to human 5HT1B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 127nMAssay Description:Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell lineMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:Binding affinity to 5HT2A receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibition of human neurokinin NK2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 332nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 521nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 525nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 525nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 714nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: 850nMAssay Description:Inhibition of human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.13E+3nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell lineMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity towards progesterone receptor was measuredMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Antagonist activity against progesterone receptor (PR) in an alkaline phosphatase assay in the T47D human breast carcinoma cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at progesterone receptor assessed as progesterone-induced alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of progesterone receptor mediated progesterone-induced alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Antagonistic activity against Progesterone receptor (PR) in transcriptional activation assay in human T47D breast carcinoma cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity against progesterone receptor in human T47D cells by alkaline phosphatase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at progesterone receptor by cellular reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity against the Progesterone Receptor (PR)More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity against progesterone receptor (PR) using PRE-luciferase plasmid co-transfected CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Binding affinity to cytosolic PR in T47D cells by competition binding assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK3(Homo sapiens (Human))
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of JAK3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Antagonist activity at glucocorticoid receptor by Gal4-DNA binding domain-hormone receptor LBD one-hybrid assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Antagonist activity at cloned glucocorticoid receptor-ligand binding domain expressed in african green monkey COS7 cells by GAL4 luciferase reporter ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK1(Homo sapiens (Human))
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of JAK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Antagonist activity at human GR ligand binding domain expressed in african green monkey COS7 cells in presence of Dexamethasone by Gal4 hybrid assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK2(Homo sapiens (Human))
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity against the Glucocorticoid Receptor (GR)More data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)