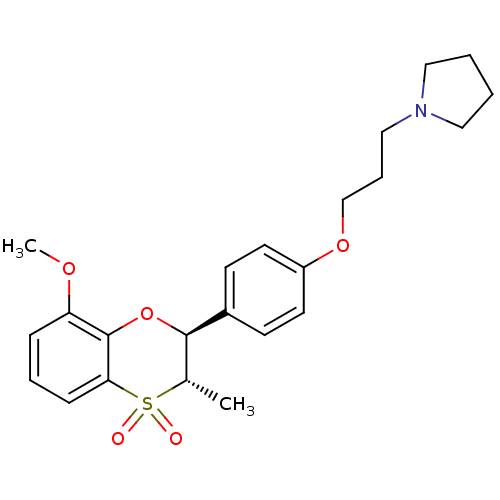
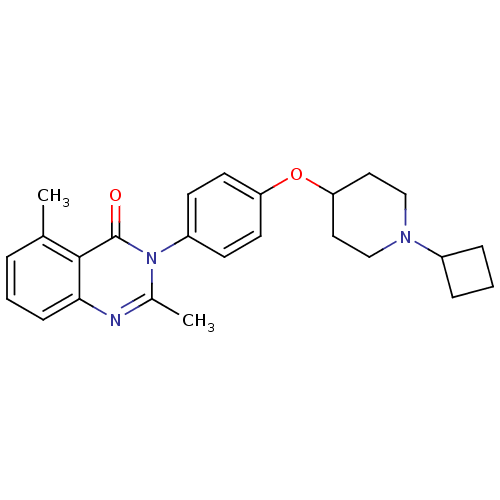
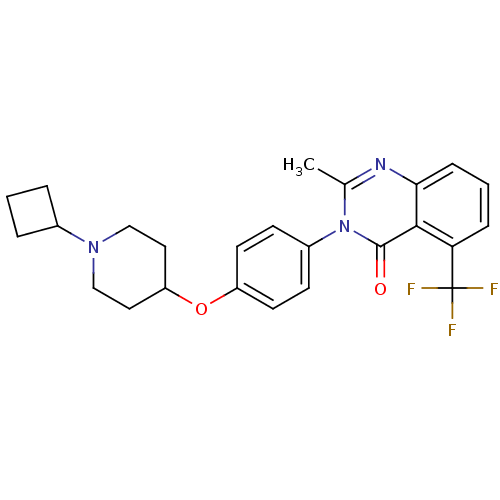
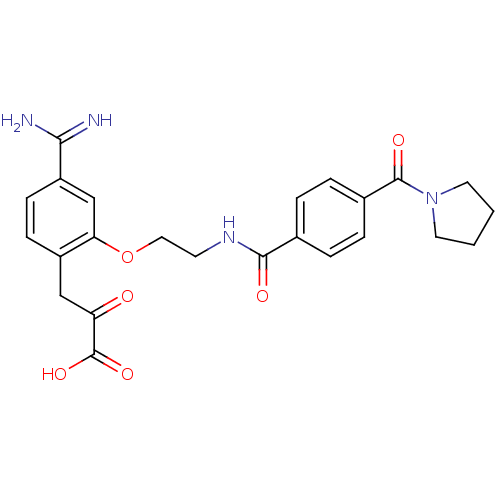
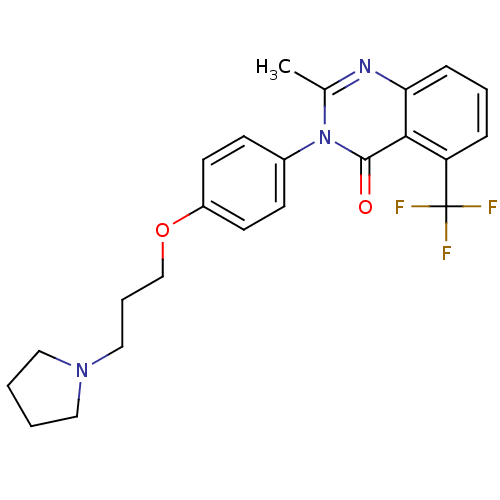
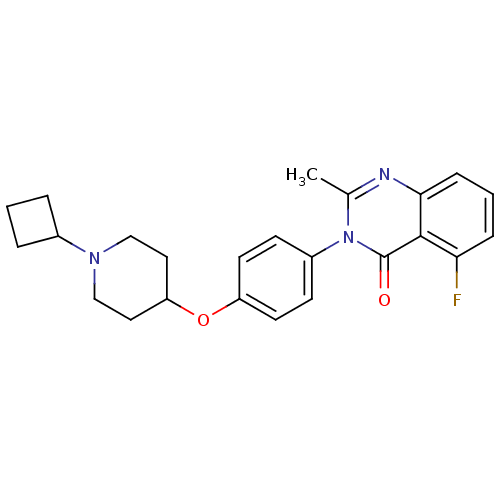
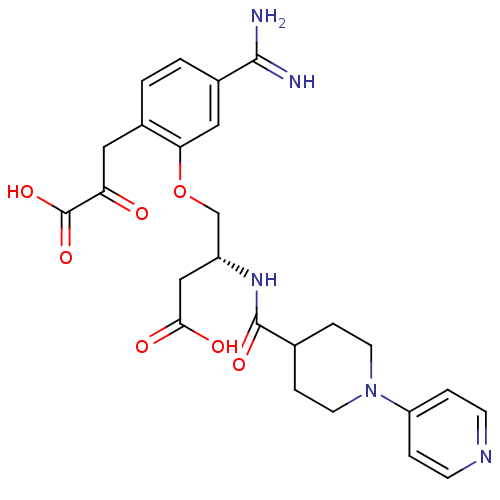
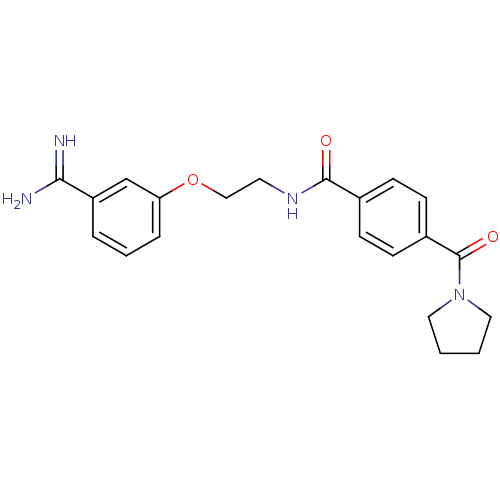
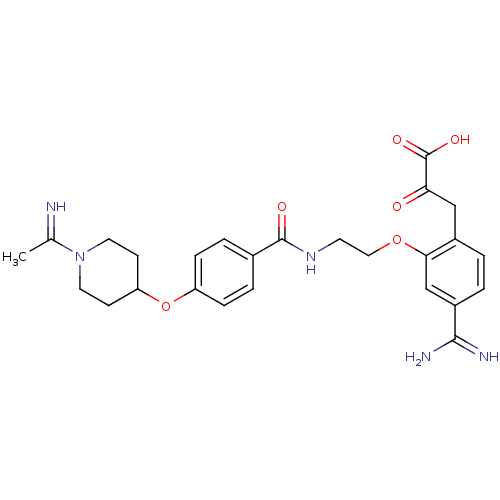
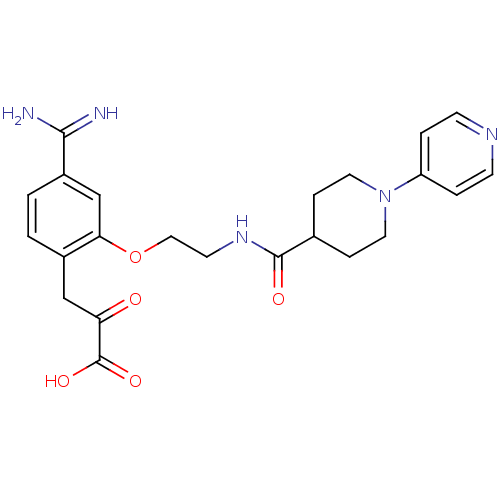
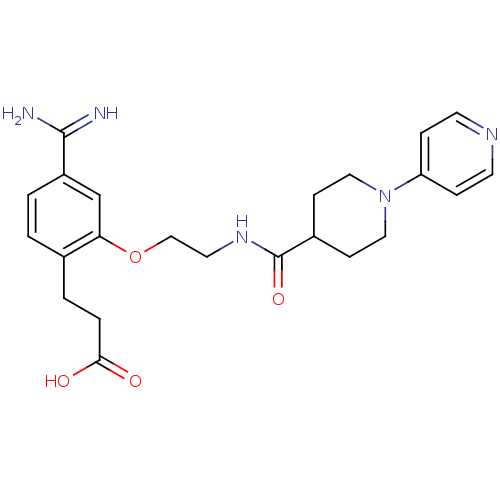
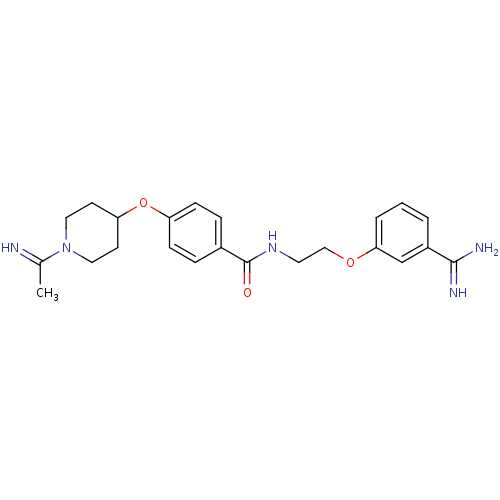
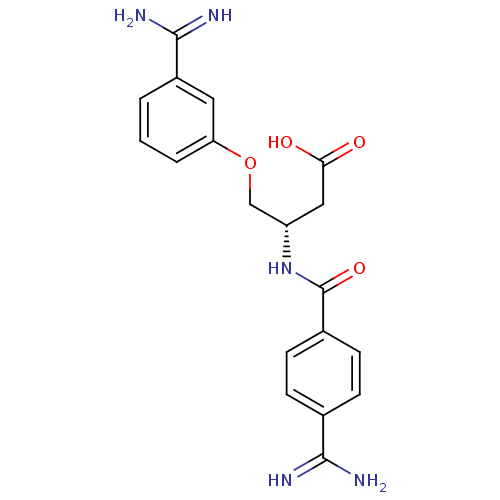
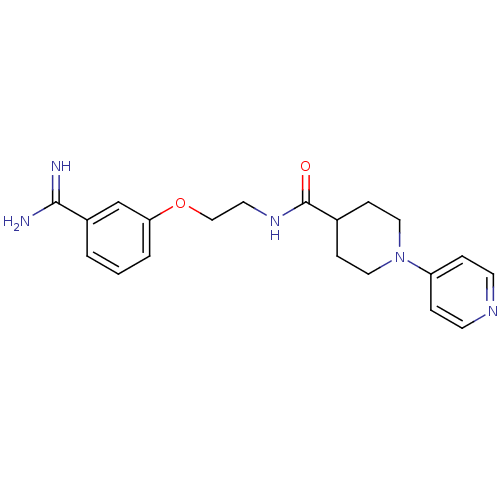
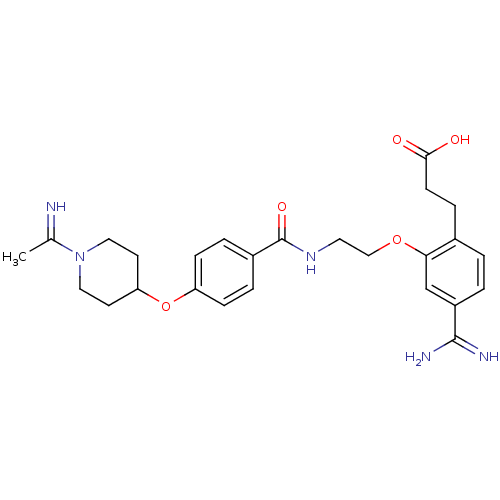
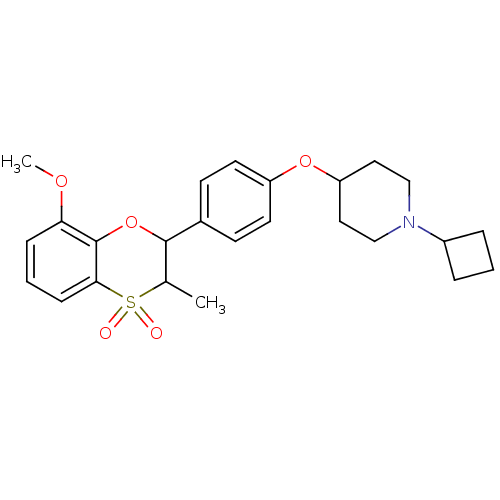
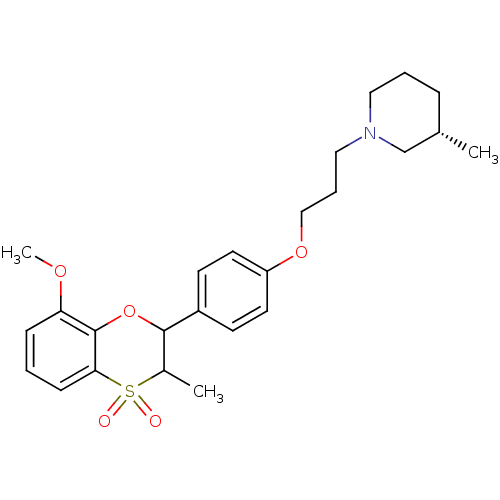
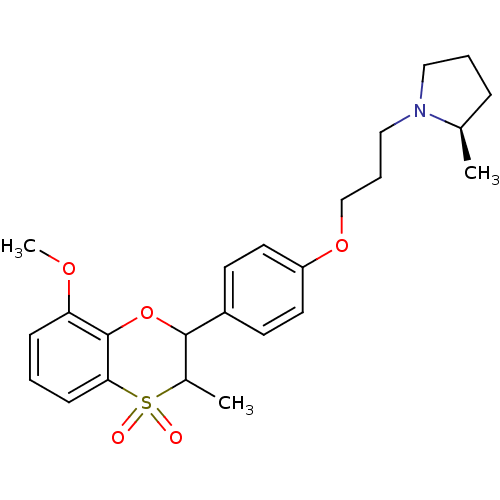
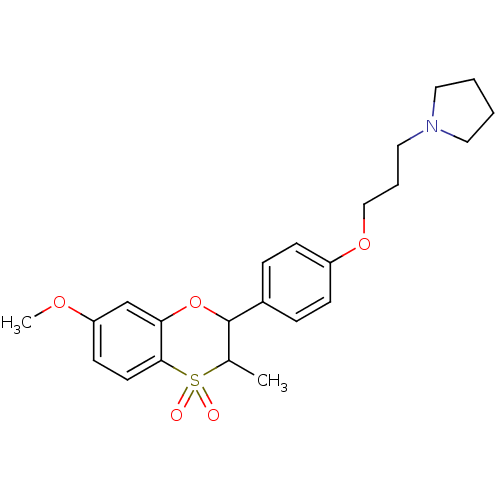
Affinity DataKi: 1nMAssay Description:Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine receptor H3(Macaca mulatta (Rhesus macaque))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8.40nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 7.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair