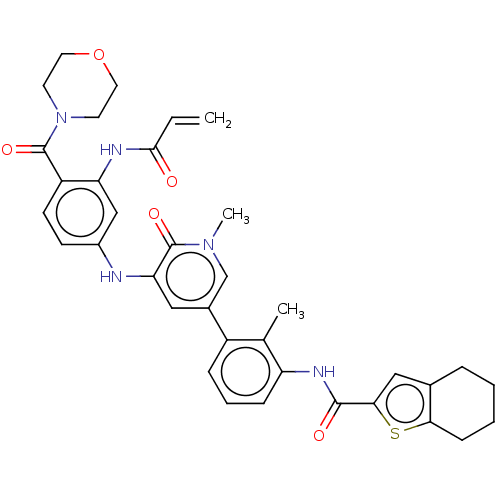
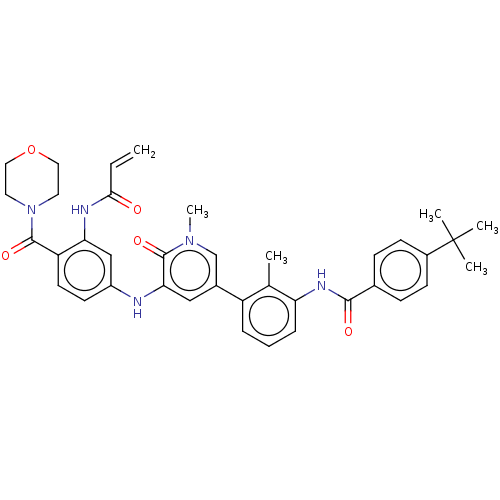
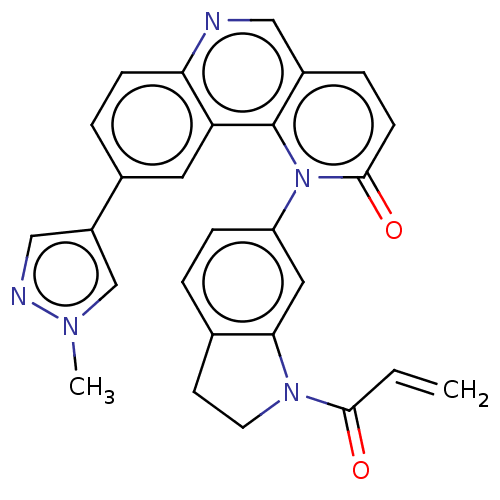
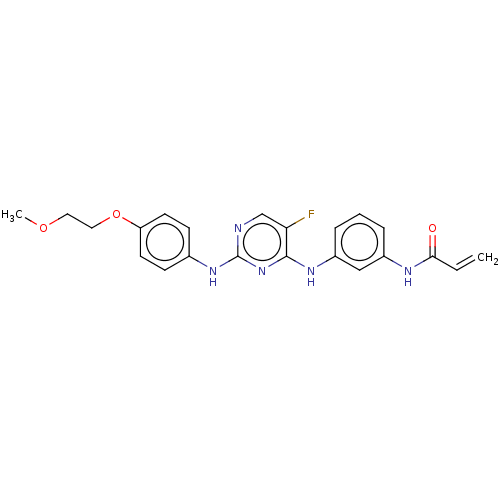
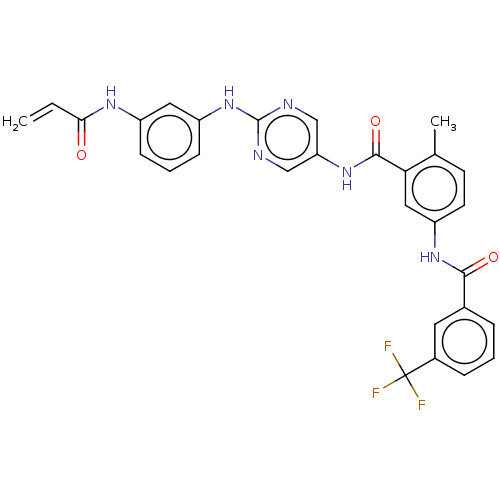
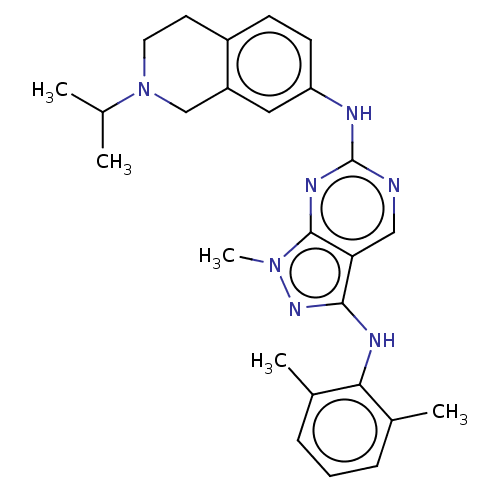
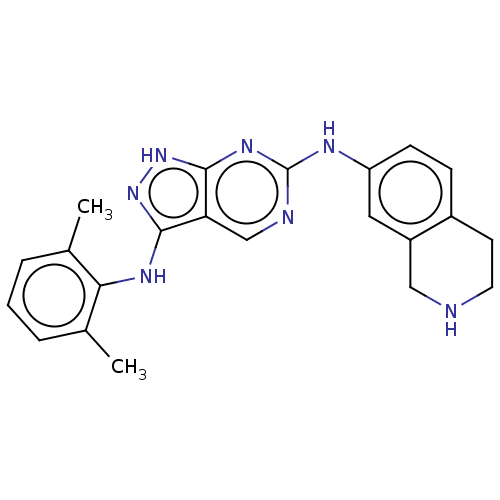
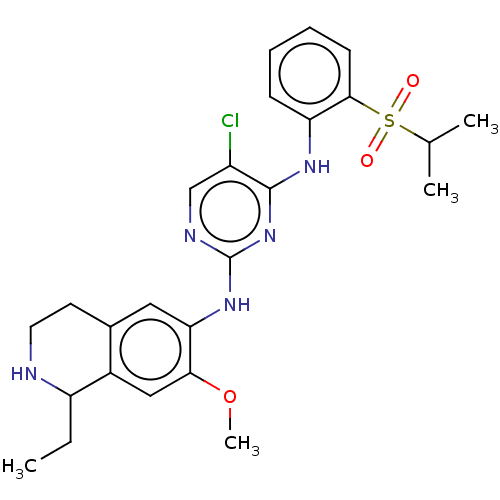
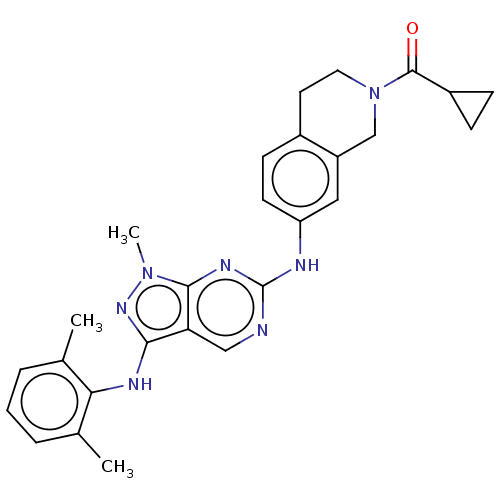
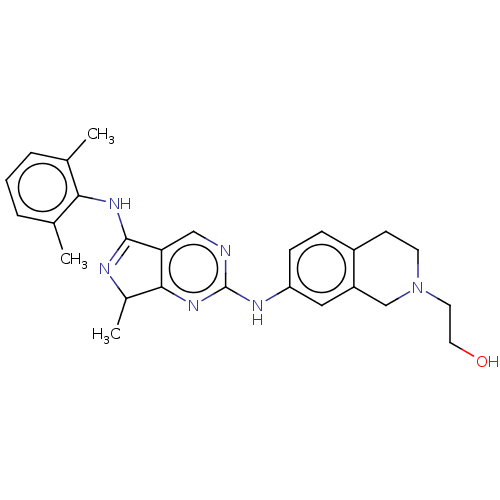
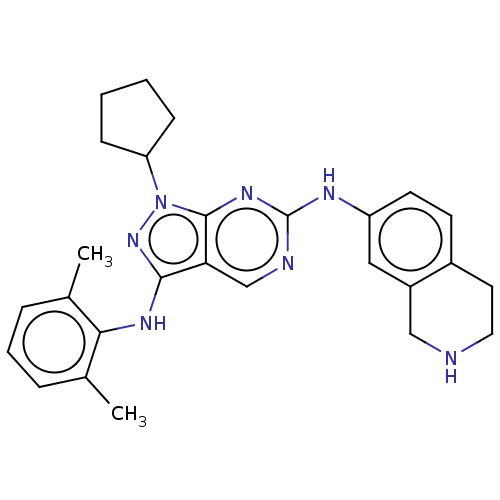
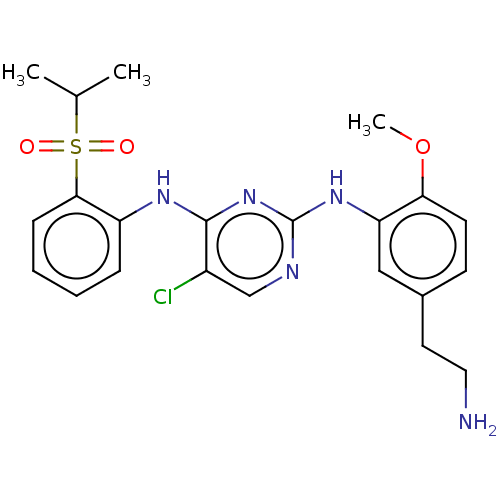
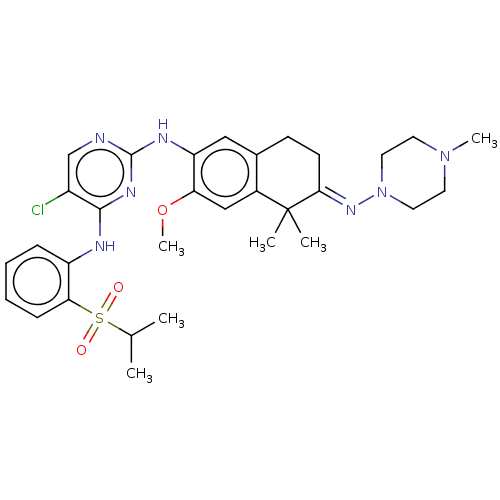
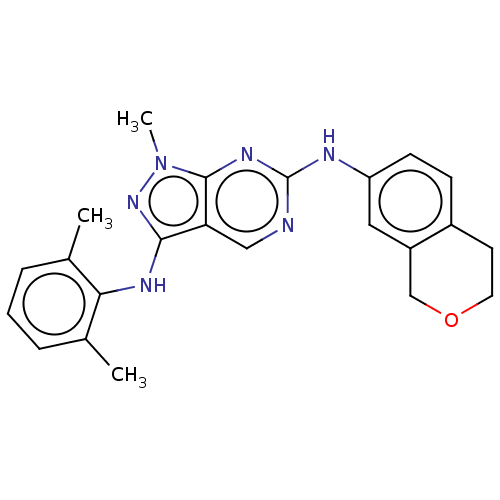
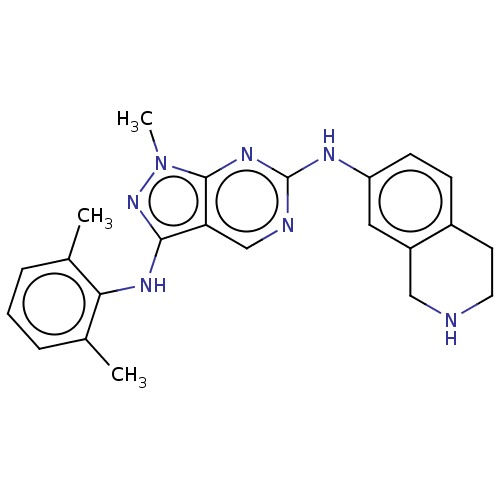
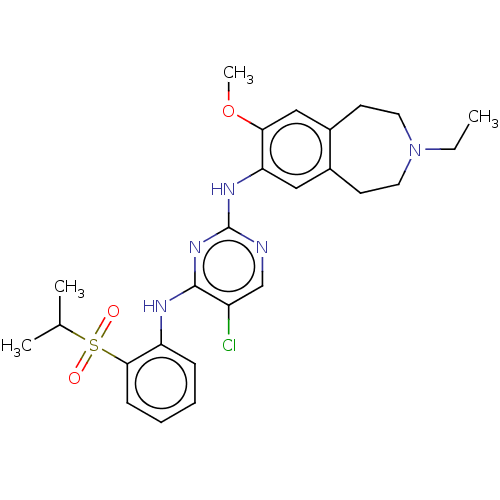
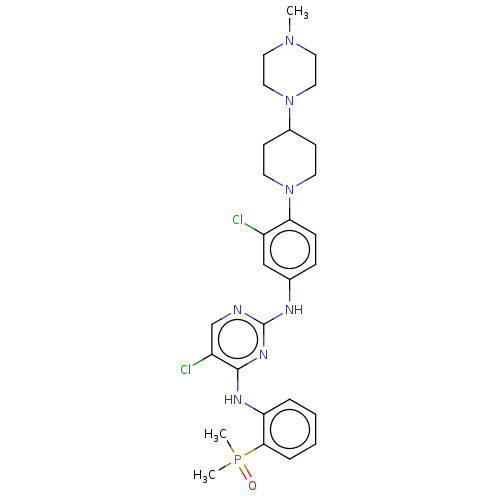
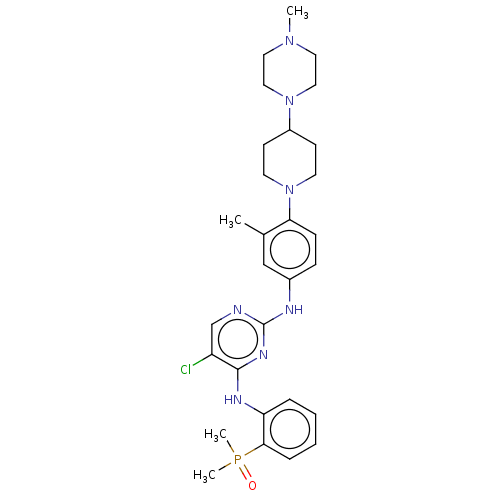
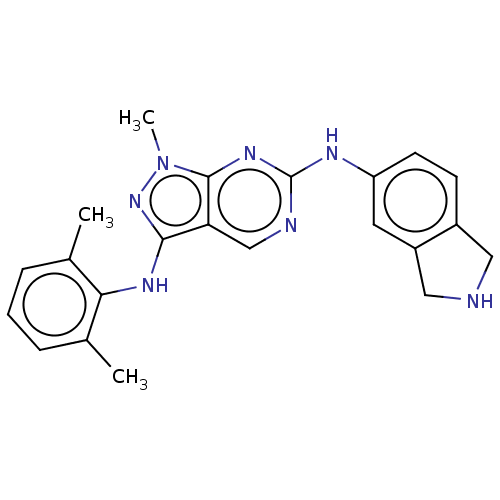
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 4.80nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
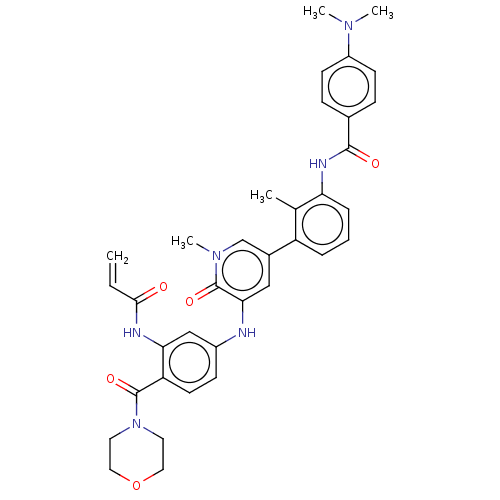
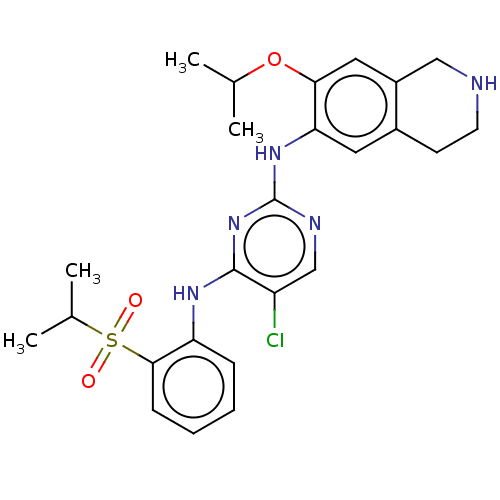
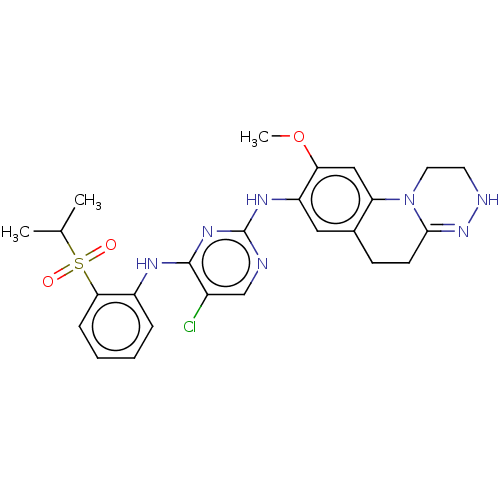
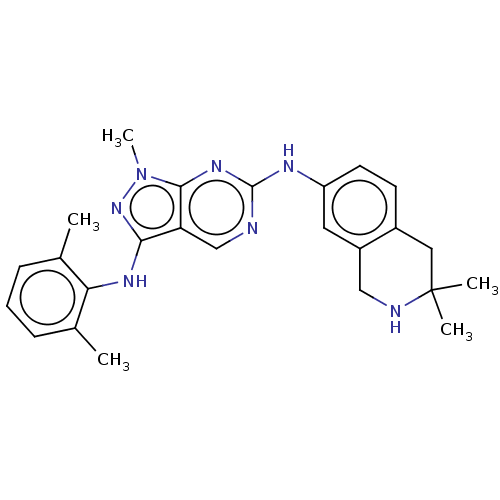
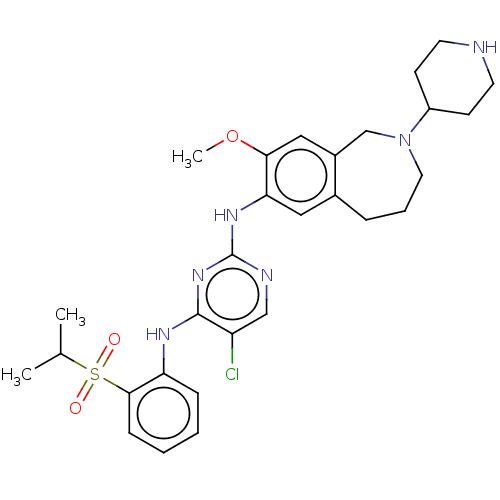
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
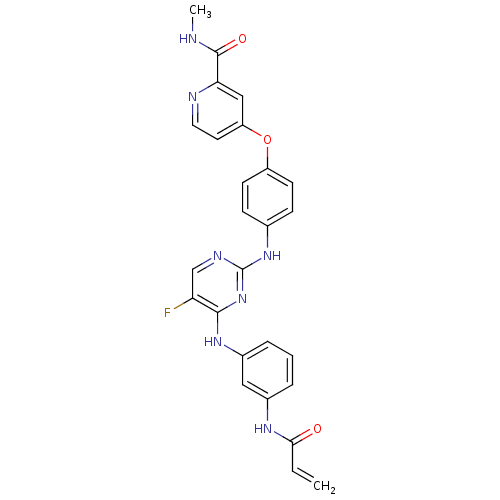
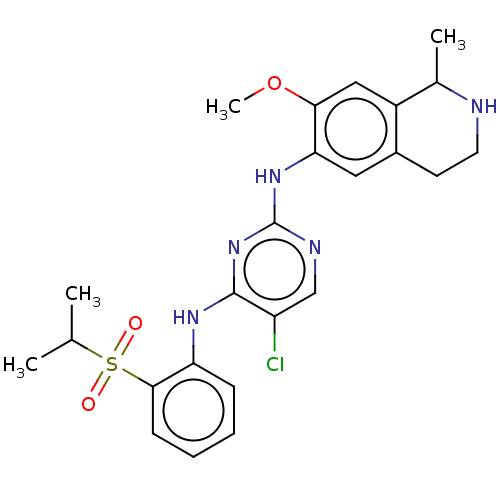
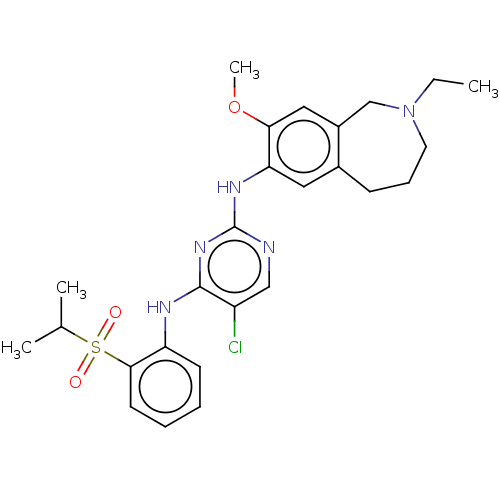
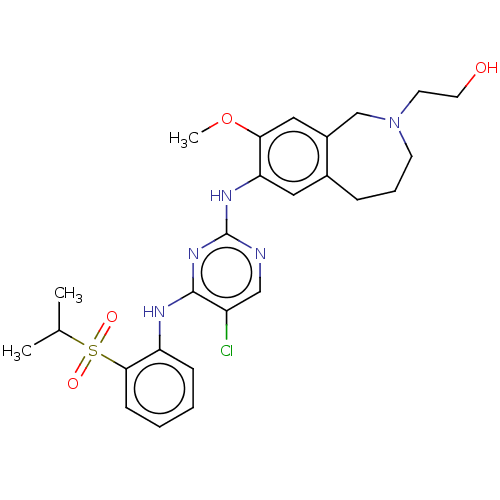
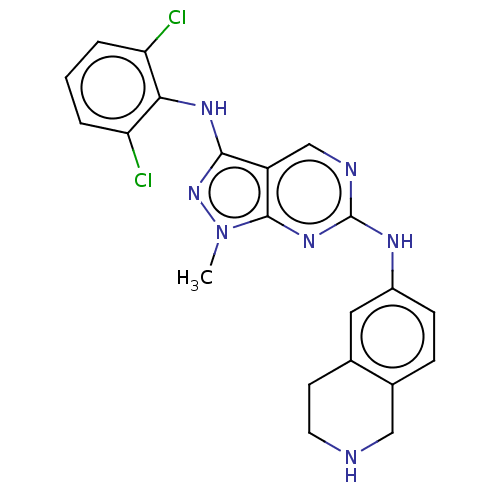
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 8.90nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
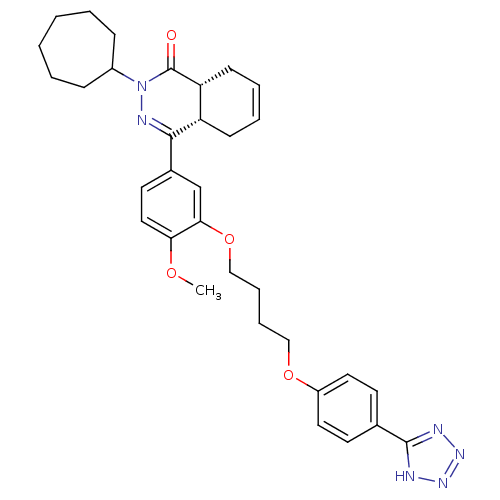
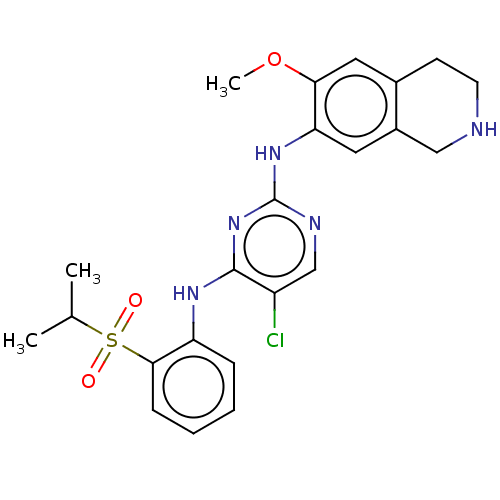
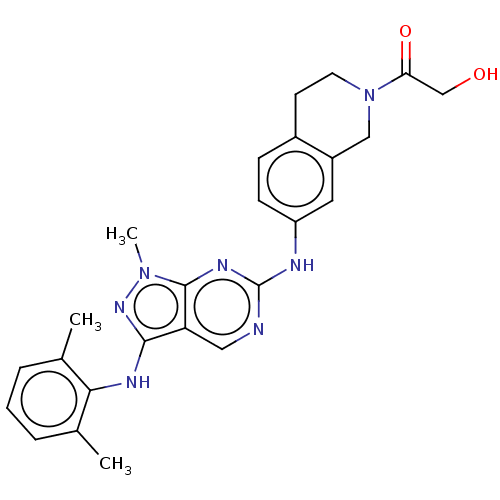
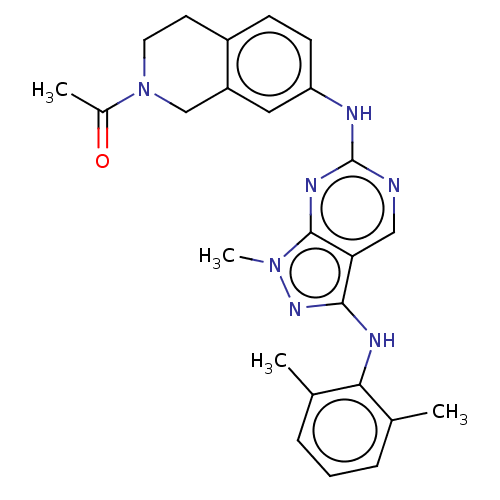
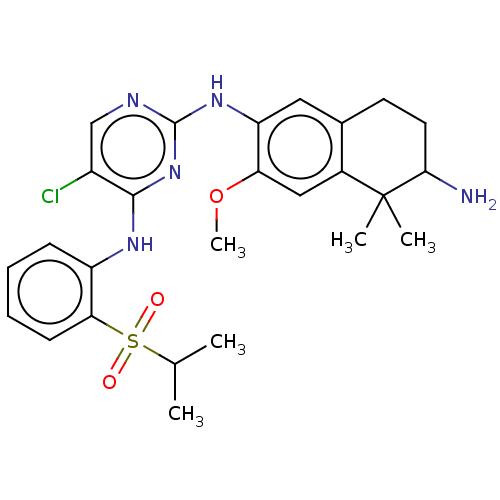
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 113nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataKi: 209nMAssay Description:Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T...More data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Mixed type inhibition of human DNMT3A using AdoMet as substrateMore data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 5.03E+3nMAssay Description:Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using 3H-AdoMet as substrate incubated fo...More data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 9.16E+3nMAssay Description:Uncompetitive inhibition of human DNMT3A using AdoMet as substrateMore data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 1.14E+4nMAssay Description:Uncompetitive inhibition of human DNMT3A using poly dl-dC as substrateMore data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 1.26E+4nMAssay Description:Mixed type inhibition of human DNMT3A using poly dl-dC as substrateMore data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 1.38E+4nMAssay Description:Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using 3H-AdoMet as substrate incubated fo...More data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 1.61E+4nMAssay Description:Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using poly dl-dC as substrate incubated f...More data for this Ligand-Target Pair
TargetDNA (cytosine-5)-methyltransferase 3A(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 2.16E+4nMAssay Description:Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using poly dl-dC as substrate incubated f...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.210nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.240nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.240nMAssay Description:Inhibition of crizotinib-resistant ALK G1269A mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor [L1196M](Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.330nMAssay Description:In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes.More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.380nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.440nMT: 2°CAssay Description:The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.450nMAssay Description:Inhibition of crizotinib-resistant ALK C1156Y mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.460nMAssay Description:Inhibition of crizotinib-resistant ALK F1174L mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.5nMAssay Description:Inhibition of wild type ALK (unknown origin) after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.520nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.523nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.540nMAssay Description:Inhibition of ALK (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescence assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.550nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of EGFR L858R/T790M/C797S triple mutant (unknown origin) measured by ELISAMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.660nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.670nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.700nMAssay Description:Inhibition of wild type ALK (unknown origin) after 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of EGFR L858R/T790M/C797S triple mutant (unknown origin) measured by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of EGFR L858R/T790M/C797S triple mutant (unknown origin) measured by ELISAMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor [L1196M](Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
University of Science and Technology of China
Curated by ChEMBL
University of Science and Technology of China
Curated by ChEMBL
Affinity DataIC50: 0.745nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)