 US11767305, Compound (-)-BK-5-MAPB BDBM620429 US20250019357, Compound (-)-BK-5MAPB
US11767305, Compound (-)-BK-5-MAPB BDBM620429 US20250019357, Compound (-)-BK-5MAPB US11767305, Compound (-)-BK-6-MAPB BDBM620431 US20250019357, Compound (-)-BK-6MAPB
US11767305, Compound (-)-BK-6-MAPB BDBM620431 US20250019357, Compound (-)-BK-6MAPB US20250019357, Compound (+)-BK-5MAPB BDBM620430 US11767305, Compound (+)-BK-5-MAPB
US20250019357, Compound (+)-BK-5MAPB BDBM620430 US11767305, Compound (+)-BK-5-MAPB US20250019357, Compound (+)-BK-6MAPB BDBM620432 US11767305, Compound (+)-BK-6-MAPB
US20250019357, Compound (+)-BK-6MAPB BDBM620432 US11767305, Compound (+)-BK-6-MAPB BDBM293152 US10106501, Example BK
BDBM293152 US10106501, Example BK US8846656, 17-BK BDBM134489
US8846656, 17-BK BDBM134489 CAS_58-82-2 NSC_105044 Bradykinin BDBM82076
CAS_58-82-2 NSC_105044 Bradykinin BDBM82076 BDBM712824 US20250019357, Compound (-)-BK-6MBPB
BDBM712824 US20250019357, Compound (-)-BK-6MBPB US20250019357, Compound (+)-BK-6MBPB BDBM712825
US20250019357, Compound (+)-BK-6MBPB BDBM712825 BDBM712809 US20250019357, Compound (+)-BK-6-MBPB
BDBM712809 US20250019357, Compound (+)-BK-6-MBPB US20250019357, Compound (-)-BK-6-MBPB BDBM712807
US20250019357, Compound (-)-BK-6-MBPB BDBM712807 BDBM712800 US20250019357, Compound rac-BK-6-MAPB
BDBM712800 US20250019357, Compound rac-BK-6-MAPB BDBM712806 US20250019357, Compound rac-BK-6-MBPB
BDBM712806 US20250019357, Compound rac-BK-6-MBPB US20250019357, Compound rac-BK-5-MAPB BDBM712797
US20250019357, Compound rac-BK-5-MAPB BDBM712797 US20250019357, Compound rac-BK-5-MBPB BDBM712803
US20250019357, Compound rac-BK-5-MBPB BDBM712803 BDBM620426 US11767305, Compound R-5-MBPB US20250019357, Compound (-)-BK-5MBPB
BDBM620426 US11767305, Compound R-5-MBPB US20250019357, Compound (-)-BK-5MBPB US20250019357, Compound (+)-BK-5MBPB BDBM620425 US11767305, Compound S-5-MBPB
US20250019357, Compound (+)-BK-5MBPB BDBM620425 US11767305, Compound S-5-MBPB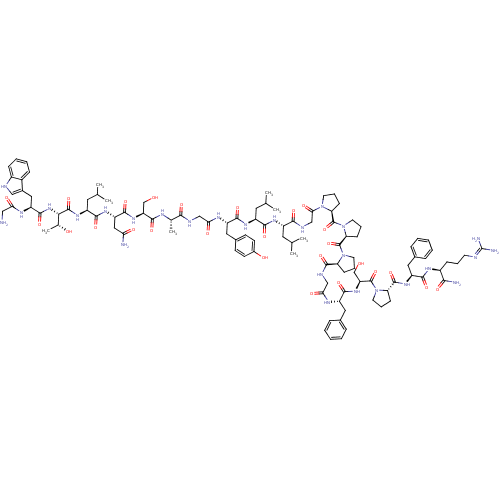 Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35
Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35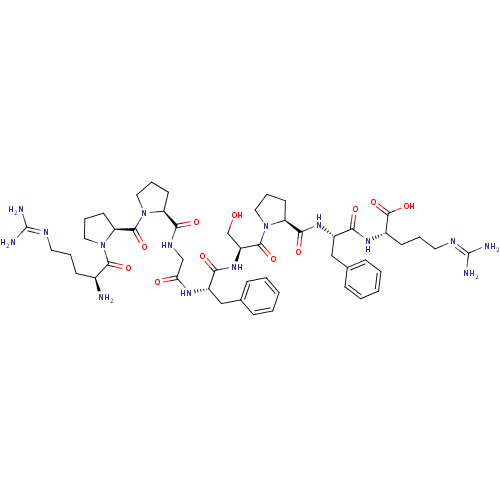 (bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)
(bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)
- Dankwardt, SM; Ferla, S; Krstenansky, JL; Bhakta, S; Ostrelich, H; Jarnagin, K Nonpeptide bradykinin antagonist analogs based on a model of a Sterling-Winthrop nonpeptide bradykinin antagonist overlapped with cyclic hexapeptide bradykinin antagonist peptides Bioorg Med Chem Lett 7: 1921-1926 (1997)
- Qiu, Y; Taichi, M; Wei, N; Yang, H; Luo, KQ; Tam, JP An Orally Active Bradykinin B J Med Chem 60: 504-510 (2017)
- Barth, M; Bondoux, M; Luccarini, JM; Peyrou, V; Dodey, P; Pruneau, D; Massardier, C; Paquet, JL From bradykinin B2 receptor antagonists to orally active and selective bradykinin B1 receptor antagonists. J Med Chem 55: 2574-84 (2012)
- Kuduk, SD; Ng, C; Feng, DM; Wai, JM; Chang, RS; Harrell, CM; Murphy, KL; Ransom, RW; Reiss, D; Ivarsson, M; Mason, G; Boyce, S; Tang, C; Prueksaritanont, T; Freidinger, RM; Pettibone, DJ; Bock, MG 2,3-diaminopyridine bradykinin B1 receptor antagonists. J Med Chem 47: 6439-42 (2004)
- Bryan, MC; Biswas, K; Peterkin, TA; Rzasa, RM; Arik, L; Lehto, SG; Sun, H; Hsieh, FY; Xu, C; Fremeau, RT; Allen, JR Chromenones as potent bradykinin B1 antagonists. Bioorg Med Chem Lett 22: 619-22 (2011)
- Kuduk, SD; Di Marco, CN; Chang, RK; Wood, MR; Kim, JJ; Schirripa, KM; Murphy, KL; Ransom, RW; Tang, C; Torrent, M; Ha, S; Prueksaritanont, T; Pettibone, DJ; Bock, MG 5-Piperazinyl pyridine carboxamide bradykinin B1 antagonists. Bioorg Med Chem Lett 16: 2791-5 (2006)
- Gibson, C; Schnatbaum, K; Pfeifer, JR; Locardi, E; Paschke, M; Reimer, U; Richter, U; Scharn, D; Faussner, A; Tradler, T Novel small molecule bradykinin B2 receptor antagonists. J Med Chem 52: 4370-9 (2009)
- Coi, A; Fiamingo, FL; Livi, O; Calderone, V; Martelli, A; Massarelli, I; Bianucci, AM QSAR studies on BK channel activators. Bioorg Med Chem 17: 319-25 (2008)
- Wood, MR; Kim, JJ; Han, W; Dorsey, BD; Homnick, CF; DiPardo, RM; Kuduk, SD; MacNeil, T; Murphy, KL; Lis, EV; Ransom, RW; Stump, GL; Lynch, JJ; O'Malley, SS; Miller, PJ; Chen, TB; Harrell, CM; Chang, RS; Sandhu, P; Ellis, JD; Bondiskey, PJ; Pettibone, DJ; Freidinger, RM; Bock, MG Benzodiazepines as potent and selective bradykinin B1 antagonists. J Med Chem 46: 1803-6 (2003)
- Bodmer-Narkevitch, V; Anthony, NJ; Cofre, V; Jolly, SM; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG; Kuduk, SD Indazole derivatives as novel bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 20: 7011-4 (2010)
- Cole, AG; Metzger, A; Brescia, MR; Qin, LY; Appell, KC; Brain, CT; Hallett, A; Ganju, P; Denholm, AA; Wareing, JR; Ritchie, TJ; Drake, GM; Bevan, SJ; MacGloinn, A; McBryde, A; Patel, V; Oakley, PJ; Nunez, X; Gstach, H; Schneider, P; Baldwin, JJ; Dolle, RE; McDonald, E; Henderson, I Sulfonamido-aryl ethers as bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 19: 119-22 (2008)
- Douty, BD; Salvino, JM; Seoane, PR; Dolle, RE Synthesis of non-peptide bradykinin B2 receptor antagonists Bioorg Med Chem Lett 5: 363-366 (1995)
- Dziadulewicz, EK; Ritchie, TJ; Hallett, A; Snell, CR; Davies, JW; Wrigglesworth, R; Dunstan, AR; Bloomfield, GC; Drake, GS; McIntyre, P; Brown, MC; Burgess, GM; Lee, W; Davis, C; Yaqoob, M; Phagoo, SB; Phillips, E; Perkins, MN; Campbell, EA; Davis, AJ; Rang, HP Nonpeptide bradykinin B2 receptor antagonists: conversion of rodent-selective bradyzide analogues into potent, orally-active human bradykinin B2 receptor antagonists. J Med Chem 45: 2160-72 (2002)
- Lee, J; Reynolds, C; Jetter, MC; Youngman, MA; Hlasta, DJ; Dax, SL; Stone, DJ; Zhang, SP; Codd, EE Design and synthesis of novel pyrrolidine-containing bradykinin antagonists. Bioorg Med Chem Lett 13: 1879-82 (2003)
- Su, DS; Lim, JL; Markowitz, MK; Wan, BL; Murphy, KL; Reiss, DR; Harrell, CM; O'Malley, SS; Ransom, RW; Chang, RS; Pettibone, DJ; Tang, C; Prueksaritanont, T; Freidinger, RM; Bock, MG Potent bradykinin B1 receptor antagonists: 4-substituted phenyl cyclohexanes. Bioorg Med Chem Lett 17: 3006-9 (2007)
- Eles, J; Beke, G; Vágó, I; Bozó, E; Huszár, J; Tarcsay, A; Kolok, S; Schmidt, E; Vastag, M; Hornok, K; Farkas, S; Domány, G; Keseru, GM Quinolinyl- and phenantridinyl-acetamides as bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 22: 3095-9 (2012)
- Maggiora, LL; Orawski, AT; Simmons, WH Apstatin analogue inhibitors of aminopeptidase P, a bradykinin-degrading enzyme. J Med Chem 42: 2394-402 (1999)
- Kuduk, SD; Chang, RK; Ng, C; Murphy, KL; Ransom, RW; Tang, C; Prueksaritanont, T; Freidinger, RM; Pettibone, DJ; Bock, MG Bradykinin B1 antagonists: SAR studies in the 2,3-diaminopyridine series. Bioorg Med Chem Lett 15: 3925-9 (2005)
- Kuduk, SD; DiPardo, RM; Chang, RK; Di Marco, CN; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG Bradykinin B1 antagonists: biphenyl SAR studies in the cyclopropanecarboxamide series. Bioorg Med Chem Lett 17: 3608-12 (2007)
- Huang, H; Player, MR Bradykinin B1 receptor antagonists as potential therapeutic agents for pain. J Med Chem 53: 5383-99 (2010)
- BAEURLE, S; DAVENPORT, A; STIMSON, C; NAGEL, J; SCHMIDT, N; ROTGERI, A; GROETICKE, I; RAUSCH, A; KLAR, J; DYRKS, T CARBOXYLIC ACID AROMATIC AMIDES AS ANTAGONISTS OF BRADYKININ B1 RECEPTOR US Patent US20230271931 (2023)
- Thurieau, C; Félétou, M; Hennig, P; Raimbaud, E; Canet, E; Fauchère, JL Design and synthesis of new linear and cyclic bradykinin antagonists. J Med Chem 39: 2095-101 (1996)
- Biswas, K; Peterkin, TA; Bryan, MC; Arik, L; Lehto, SG; Sun, H; Hsieh, FY; Xu, C; Fremeau, RT; Allen, JR Discovery of potent, orally bioavailable phthalazinone bradykinin B1 receptor antagonists. J Med Chem 54: 7232-46 (2011)
- Salvino, JM; Seoane, PR; Douty, BD; Awad, MA; Hoyer, D; Ross, TM; Dolle, RE; Houck, WT; Faunce, DM; Sawutz, DG Structure activity relationships of non-peptide bradykinin B2 receptor antagonists Bioorg Med Chem Lett 5: 357-362 (1995)
- Su, DS; Lim, JL; Tinney, E; Wan, BL; Murphy, KL; Reiss, DR; Harrell, CM; O'Malley, SS; Ransom, RW; Chang, RS; Pettibone, DJ; Yu, J; Tang, C; Prueksaritanont, T; Freidinger, RM; Bock, MG; Anthony, NJ 2-Aminobenzophenones as a novel class of bradykinin B1 receptor antagonists. J Med Chem 51: 3946-52 (2008)
- Amblard, M; Daffix, I; Bedos, P; Bergé, G; Pruneau, D; Paquet, JL; Luccarini, JM; Bélichard, P; Dodey, P; Martinez, J Design and synthesis of potent bradykinin agonists containing a benzothiazepine moiety. J Med Chem 42: 4185-92 (1999)
- Schnatbaum, K; Schaudt, M; Stragies, R; Pfeifer, JR; Gibson, C; Locardi, E; Scharn, D; Richter, U; Kalkhof, H; Dinkel, K; Zischinsky, G Novel small molecule bradykinin B1 receptor antagonists. Part 3: hydroxyurea derivatives. Bioorg Med Chem Lett 20: 1233-6 (2010)
- Youngman, MA; Carson, JR; Lee, JS; Dax, SL; Zhang, SP; Colburn, RW; Stone, DJ; Codd, EE; Jetter, MC Synthesis and structure--activity relationships of aroylpyrrole alkylamide bradykinin (B2) antagonists. Bioorg Med Chem Lett 13: 1341-4 (2003)
- Wood, MR; Schirripa, KM; Kim, JJ; Kuduk, SD; Chang, RK; Di Marco, CN; DiPardo, RM; Wan, BL; Murphy, KL; Ransom, RW; Chang, RS; Holahan, MA; Cook, JJ; Lemaire, W; Mosser, SD; Bednar, RA; Tang, C; Prueksaritanont, T; Wallace, AA; Mei, Q; Yu, J; Bohn, DL; Clayton, FC; Adarayn, ED; Sitko, GR; Leonard, YM; Freidinger, RM; Pettibone, DJ; Bock, MG Alpha-hydroxy amides as a novel class of bradykinin B1 selective antagonists. Bioorg Med Chem Lett 18: 716-20 (2008)
- Schaudt, M; Locardi, E; Zischinsky, G; Stragies, R; Pfeifer, JR; Gibson, C; Scharn, D; Richter, U; Kalkhof, H; Dinkel, K; Schnatbaum, K Novel small molecule bradykinin B1 receptor antagonists. Part 1: benzamides and semicarbazides. Bioorg Med Chem Lett 20: 1225-8 (2010)
- Zischinsky, G; Stragies, R; Schaudt, M; Pfeifer, JR; Gibson, C; Locardi, E; Scharn, D; Richter, U; Kalkhof, H; Dinkel, K; Schnatbaum, K Novel small molecule bradykinin B1 receptor antagonists. Part 2: 5-membered diaminoheterocycles. Bioorg Med Chem Lett 20: 1229-32 (2010)
- Stahl, W; Breipohl, G; Kuhlmann, L; Steinsträsser, A; Gerhards, HJ; Schölkens, BA Technetium-99m-labeled HOE 140: a potential bradykinin B2 receptor imaging agent. J Med Chem 38: 2799-801 (1995)
- Liu, X; Tao, J; Zhang, S; Lan, W; Wang, C; Ji, Y; Cao, C Selective Blockade of Neuronal BK (α + β4) Channels Preventing Epileptic Seizure. J Med Chem 63: 216-230 (2020)
- Hoyer, D; Awad, MM; Salvino, JM; Seoane, PR; Dolle, RE; Houck, WT; Sawutz, DG Ace inhibitors as a template for the design of bradykinin B2 receptor antagonists Bioorg Med Chem Lett 5: 367-370 (1995)
- Biswas, K; Aya, T; Qian, W; Peterkin, TA; Chen, JJ; Human, J; Hungate, RW; Kumar, G; Arik, L; Lester-Zeiner, D; Biddlecome, G; Manning, BH; Sun, H; Dong, H; Huang, M; Loeloff, R; Johnson, EJ; Askew, BC Aryl sulfones as novel bradykinin B1 receptor antagonists for treatment of chronic pain. Bioorg Med Chem Lett 18: 4764-9 (2008)
- Kuduk, SD; Chang, RK; Dipardo, RM; Di Marco, CN; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG Bradykinin B1 receptor antagonists: an alpha-hydroxy amide with an improved metabolism profile. Bioorg Med Chem Lett 18: 5107-10 (2008)
- Salvino, JM; Seoane, PR; Douty, BD; Awad, MM; Dolle, RE; Houck, WT; Faunce, DM; Sawutz, DG Design of potent non-peptide competitive antagonists of the human bradykinin B2 receptor. J Med Chem 36: 2583-4 (1993)
- Chakravarty, S; Wilkins, D; Kyle, DJ Design of potent, cyclic peptide bradykinin receptor antagonists from conformationally constrained linear peptides. J Med Chem 36: 2569-71 (1993)
- Su, DS; Markowitz, MK; Murphy, KL; Wan, BL; Zrada, MM; Harrell, CM; O'Malley, SS; Hess, JF; Ransom, RW; Chang, RS; Wallace, MA; Raab, CE; Dean, DC; Pettibone, DJ; Freidinger, RM; Bock, MG Development of an efficient and selective radioligand for bradykinin B1 receptor occupancy studies. Bioorg Med Chem Lett 14: 6045-8 (2004)
- Kuduk, SD; Di Marco, CN; Chang, RK; Wood, MR; Schirripa, KM; Kim, JJ; Wai, JM; DiPardo, RM; Murphy, KL; Ransom, RW; Harrell, CM; Reiss, DR; Holahan, MA; Cook, J; Hess, JF; Sain, N; Urban, MO; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG Development of orally bioavailable and CNS penetrant biphenylaminocyclopropane carboxamide bradykinin B1 receptor antagonists. J Med Chem 50: 272-82 (2007)
- Chen, JJ; Qian, W; Biswas, K; Viswanadhan, VN; Askew, BC; Hitchcock, S; Hungate, RW; Arik, L; Johnson, E Discovery of dihydroquinoxalinone acetamides containing bicyclic amines as potent Bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 18: 4477-81 (2008)
- Amblard, M; Daffix, I; Bergé, G; Calmès, M; Dodey, P; Pruneau, D; Paquet, JL; Luccarini, JM; Bélichard, P; Martinez, J Synthesis and characterization of bradykinin B(2) receptor agonists containing constrained dipeptide mimics. J Med Chem 42: 4193-201 (1999)
- Bonafoux, D; Nanthakumar, S; Bandarage, UK; Memmott, C; Lowe, D; Aronov, AM; Bhisetti, GR; Bonanno, KC; Coll, J; Leeman, J; Lepre, CA; Lu, F; Perola, E; Rijnbrand, R; Taylor, WP; Wilson, D; Zhou, Y; Zwahlen, J; Ter Haar, E Fragment-Based Discovery of Dual JC Virus and BK Virus Helicase Inhibitors. J Med Chem 59: 7138-51 (2016)
- Guo, Q; Chandrasekhar, J; Ihle, D; Wustrow, DJ; Chenard, BL; Krause, JE; Hutchison, A; Alderman, D; Cheng, C; Cortright, D; Broom, D; Kershaw, MT; Simmermacher-Mayer, J; Peng, Y; Hodgetts, KJ 1-Benzylbenzimidazoles: the discovery of a novel series of bradykinin B(1) receptor antagonists. Bioorg Med Chem Lett 18: 5027-31 (2008)
- Feng, DM; Wai, JM; Kuduk, SD; Ng, C; Murphy, KL; Ransom, RW; Reiss, D; Chang, RS; Harrell, CM; MacNeil, T; Tang, C; Prueksaritanont, T; Freidinger, RM; Pettibone, DJ; Bock, MG 2,3-Diaminopyridine as a platform for designing structurally unique nonpeptide bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 15: 2385-8 (2005)
- Liu, Q; Qian, W; Li, A; Biswas, K; Chen, JJ; Fotsch, C; Han, N; Yuan, C; Arik, L; Biddlecome, G; Johnson, E; Kumar, G; Lester-Zeiner, D; Ng, GY; Hungate, RW; Askew, BC Aryl sulfonamides containing tetralin allylic amines as potent and selective bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 20: 4593-7 (2010)
- Deekonda, S; Rankin, D; Davis, P; Lai, J; Porreca, F; Hruby, VJ Design, synthesis and biological evaluation of multifunctional ligands targeting opioid and bradykinin 2 receptors. Bioorg Med Chem Lett 25: 4148-52 (2015)
- Huszár, J; Timár, Z; Szalai, KK; Keseru, G; Fülöp, F; Penke, B Novel bradykinin-1 antagonists containing a (1,2,3,4-tetrahydro-isoquinolin-1-yl)acetic acid scaffold. Eur J Med Chem 43: 1552-8 (2008)
- Fotsch, C; Biddlecome, G; Biswas, K; Chen, JJ; D'Amico, DC; Groneberg, RD; Han, NB; Hsieh, FY; Kamassah, A; Kumar, G; Lester-Zeiner, D; Liu, Q; Mareska, DA; Riahi, BB; Wang, YJ; Yang, K; Zhan, J; Zhu, J; Johnson, E; Ng, G; Askew, BC A new class of bradykinin 1 receptor antagonists containing the piperidine acetic acid tetralin core. Bioorg Med Chem Lett 16: 2071-5 (2006)
- D'Amico, DC; Aya, T; Human, J; Fotsch, C; Chen, JJ; Biswas, K; Riahi, B; Norman, MH; Willoughby, CA; Hungate, R; Reider, PJ; Biddlecome, G; Lester-Zeiner, D; Staden, CV; Johnson, E; Kamassah, A; Arik, L; Wang, J; Viswanadhan, VN; Groneberg, RD; Zhan, J; Suzuki, H; Toro, A; Mareska, DA; Clarke, DE; Harvey, DM; Burgess, LE; Laird, ER; Askew, B; Ng, G Identification of a nonpeptidic and conformationally restricted bradykinin B1 receptor antagonist with anti-inflammatory activity. J Med Chem 50: 607-10 (2007)
- Kyle, DJ; Blake, PR; Smithwick, D; Green, LM; Martin, JA; Sinsko, JA; Summers, MF NMR and computational evidence that high-affinity bradykinin receptor antagonists adopt C-terminal beta-turns. J Med Chem 36: 1450-60 (1993)
- Dressen, D; Garofalo, AW; Hawkinson, J; Hom, D; Jagodzinski, J; Marugg, JL; Neitzel, ML; Pleiss, MA; Szoke, B; Tung, JS; Wone, DW; Wu, J; Zhang, H Preparation and optimization of a series of 3-carboxamido-5-phenacylaminopyrazole bradykinin B1 receptor antagonists. J Med Chem 50: 5161-7 (2007)
- Artis, DR; Brotherton-Pleiss, C; Pease, JH; Lin, CJ; Ferla, SW; Newman, SR; Bhakta, S; Ostrelich, H; Jarnagin, K Structure-based design of six novel classes of nonpeptide antagonists of the bradykinin B2 receptor. Bioorg Med Chem Lett 10: 2421-5 (2001)
- Dziadulewicz, EK; Brown, MC; Dunstan, AR; Lee, W; Said, NB; Garratt, PJ The design of non-peptide human bradykinin B2 receptor antagonists employing the benzodiazepine peptidomimetic scaffold. Bioorg Med Chem Lett 9: 463-8 (1999)
- Feng, DM; DiPardo, RM; Wai, JM; Chang, RK; Di Marco, CN; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG; Kuduk, SD A new class of bradykinin B1 receptor antagonists with high oral bioavailability and minimal PXR activity. Bioorg Med Chem Lett 18: 682-7 (2008)
- Bedos, P; Amblard, M; Subra, G; Dodey, P; Luccarini, JM; Paquet, JL; Pruneau, D; Aumelas, A; Martinez, J A rational approach to the design and synthesis of a new bradykinin B(1) receptor antagonist. J Med Chem 43: 2387-94 (2000)
- Qian, W; Chen, JJ; Human, J; Aya, T; Zhu, J; Biswas, K; Peterkin, T; Hungate, RW; Arik, L; Johnson, E; Kumar, G; Joseph, S; Jona, J; Guo, HX; Wu, Z Discovery of dehydro-oxopiperazine acetamides as novel bradykinin B1 receptor antagonists with enhanced in vitro potency. Bioorg Med Chem Lett 22: 1061-7 (2012)
- Biswas, K; Li, A; Chen, JJ; D'Amico, DC; Fotsch, C; Han, N; Human, J; Liu, Q; Norman, MH; Riahi, B; Yuan, C; Suzuki, H; Mareska, DA; Zhan, J; Clarke, DE; Toro, A; Groneberg, RD; Burgess, LE; Lester-Zeiner, D; Biddlecome, G; Manning, BH; Arik, L; Dong, H; Huang, M; Kamassah, A; Loeloff, R; Sun, H; Hsieh, FY; Kumar, G; Ng, GY; Hungate, RW; Askew, BC; Johnson, E Potent nonpeptide antagonists of the bradykinin B1 receptor: structure-activity relationship studies with novel diaminochroman carboxamides. J Med Chem 50: 2200-12 (2007)
- Amblard, M; Bedos, P; Olivier, C; Daffix, I; Luccarini, JM; Dodey, P; Pruneau, D; Paquet, JL; Martinez, J Synthesis and biological evaluation of bradykinin B(1)/B(2) and selective B(1) receptor antagonists. J Med Chem 43: 2382-6 (2000)
- Calderone, V; Fiamingo, FL; Amato, G; Giorgi, I; Livi, O; Martelli, A; Martinotti, E 1,2,3-Triazol-carboxanilides and 1,2,3-triazol-(N-benzyl)-carboxamides as BK-potassium channel activators. XII. Eur J Med Chem 43: 2618-26 (2008)
- Kyle, DJ; Chakravarty, S; Sinsko, JA; Stormann, TM A proposed model of bradykinin bound to the rat B2 receptor and its utility for drug design. J Med Chem 37: 1347-54 (1994)
- Guo, XM; Yadav, MB; Khan, M; Hao, CW; Lin, CY; Huang, T; Wu, J; Fan, BM; Bian, ZX Bradykinin-Potentiating Peptide-Paclitaxel Conjugate Directed at Ectopically Expressed Angiotensin-Converting Enzyme in Triple-Negative Breast Cancer. J Med Chem 64: 17051-17062 (2021)
- Tancredi, M; Galoppini, C; Meini, S; Quartara, L; Maggi, CA; Rovero, P Synthesis and biological activity of new bradykinin pseudopeptide B1 receptor agonists containing alkylic spacers Bioorg Med Chem Lett 7: 2661-2664 (1997)
- Chen, JJ; Nguyen, T; D'Amico, DC; Qian, W; Human, J; Aya, T; Biswas, K; Fotsch, C; Han, N; Liu, Q; Nishimura, N; Peterkin, TA; Yang, K; Zhu, J; Riahi, BB; Hungate, RW; Andersen, NG; Colyer, JT; Faul, MM; Kamassah, A; Wang, J; Jona, J; Kumar, G; Johnson, E; Askew, BC 3-Oxo-2-piperazinyl acetamides as potent bradykinin B1 receptor antagonists for the treatment of pain and inflammation. Bioorg Med Chem Lett 21: 3384-9 (2011)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Imai, K; Inamura, N; Asano, M; Hatori, C; Katayama, A; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 1. Construction of the basic framework. J Med Chem 41: 564-78 (1998)
- Heitsch, H; Wagner, A; Schölkens, BA; Wirth, K Novel series of O-substituted 8-quinolines and 4-benzothiazoles as potent antagonists of the bradykinin B2 receptors. Bioorg Med Chem Lett 9: 327-32 (1999)
- Rassias, G; Leonardi, S; Rigopoulou, D; Vachlioti, E; Afratis, K; Piperigkou, Z; Koutsakis, C; Karamanos, NK; Gavras, H; Papaioannou, D Potent antiproliferative activity of bradykinin B2 receptor selective agonist FR-190997 and analogue structures thereof: A paradox resolved? Eur J Med Chem 210: (2021)
- Direct Inhibition of BK Channels by Cannabidiol, One of the Principal Therapeutic Cannabinoids Derived from Cannabis sativa.
- Fincham, CI; Bressan, A; D'Andrea, P; Ettorre, A; Giuliani, S; Mauro, S; Meini, S; Paris, M; Quartara, L; Rossi, C; Squarcia, A; Valenti, C; Daniela, F; Maggi, CA Design and synthesis of novel sulfonamide-containing bradykinin hB(2) receptor antagonists. Synthesis and structure-relationships ofa,a-tetrahydropyranylglycine. Bioorg Med Chem 20: 2091-100 (2012)
- Mavunkel, BJ; Lu, Z; Goehring, RR; Lu, S; Chakravarty, S; Perumattam, J; Novotny, EA; Connolly, M; Valentine, H; Kyle, DJ Synthesis and characterization of pseudopeptide bradykinin B2 receptor antagonists containing the 1,3,8-triazaspiro[4.5]decan-4-one ring system. J Med Chem 39: 3169-73 (1996)
- Dziadulewicz, EK; Ritchie, TJ; Hallett, A; Snell, CR; Ko, SY; Wrigglesworth, R; Hughes, GA; Dunstan, AR; Bloomfield, GC; Drake, GS; Brown, MC; Lee, W; Burgess, GM; Davis, C; Yaqoob, M; Perkins, MN; Campbell, EA; Davis, AJ; Rang, HP 1-(2-Nitrophenyl)thiosemicarbazides: a novel class of potent, orally active non-peptide antagonist for the bradykinin B(2) receptor. J Med Chem 43: 769-71 (2000)
- Wood, MR; Schirripa, KM; Kim, JJ; Wan, BL; Murphy, KL; Ransom, RW; Chang, RS; Tang, C; Prueksaritanont, T; Detwiler, TJ; Hettrick, LA; Landis, ER; Leonard, YM; Krueger, JA; Lewis, SD; Pettibone, DJ; Freidinger, RM; Bock, MG Cyclopropylamino acid amide as a pharmacophoric replacement for 2,3-diaminopyridine. Application to the design of novel bradykinin B1 receptor antagonists. J Med Chem 49: 1231-4 (2006)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Inamura, N; Asano, M; Aramori, I; Hatori, C; Sawai, H; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 4. Discovery of novel frameworks mimicking the active conformation. J Med Chem 41: 4587-98 (1998)
- Goodfellow, VS; Marathe, MV; Kuhlman, KG; Fitzpatrick, TD; Cuadrado, D; Hanson, W; Zuzack, JS; Ross, SE; Wieczorek, M; Burkard, M; Whalley, ET Bradykinin receptor antagonists containing N-substituted amino acids: in vitro and in vivo B(2) and B(1) receptor antagonist activity. J Med Chem 39: 1472-84 (1996)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Inamura, N; Asano, M; Hatori, C; Sawai, H; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 2. Overcoming the species difference between guinea pig and man. J Med Chem 41: 4053-61 (1998)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Inamura, N; Asano, M; Aramori, I; Hatori, C; Sawai, H; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 3. Discovering bioisosteres of the imidazo[1,2-a] pyridine moiety. J Med Chem 41: 4062-79 (1998)
- Ritchie, TJ; Dziadulewicz, EK; Culshaw, AJ; Müller, W; Burgess, GM; Bloomfield, GC; Drake, GS; Dunstan, AR; Beattie, D; Hughes, GA; Ganju, P; McIntyre, P; Bevan, SJ; Davis, C; Yaqoob, M Potent and orally bioavailable non-peptide antagonists at the human bradykinin B(1) receptor based on a 2-alkylamino-5-sulfamoylbenzamide core. J Med Chem 47: 4642-4 (2004)
- Sawada, Y; Kayakiri, H; Abe, Y; Mizutani, T; Inamura, N; Asano, M; Hatori, C; Aramori, I; Oku, T; Tanaka, H Discovery of the first non-peptide full agonists for the human bradykinin B(2) receptor incorporating 4-(2-picolyloxy)quinoline and 1-(2-picolyl)benzimidazole frameworks. J Med Chem 47: 2853-63 (2004)
- Sawada, Y; Kayakiri, H; Abe, Y; Imai, K; Mizutani, T; Inamura, N; Asano, M; Aramori, I; Hatori, C; Katayama, A; Oku, T; Tanaka, H A new series of highly potent non-peptide bradykinin B2 receptor antagonists incorporating the 4-heteroarylquinoline framework. Improvement of aqueous solubility and new insights into species difference. J Med Chem 47: 1617-30 (2004)
- Sawada, Y; Kayakiri, H; Abe, Y; Mizutani, T; Inamura, N; Asano, M; Aramori, I; Hatori, C; Oku, T; Tanaka, H A new class of nonpeptide bradykinin B(2) receptor ligand, incorporating a 4-aminoquinoline framework. Identification of a key pharmacophore to determine species difference and agonist/antagonist profile. J Med Chem 47: 2667-77 (2004)
- Taylor, AM; Bailey, C; Belmont, LD; Campbell, R; Cantone, N; Côté, A; Crawford, TD; Cummings, R; DeMent, K; Duplessis, M; Flynn, M; Good, AC; Huang, HR; Joshi, S; Leblanc, Y; Murray, J; Nasveschuk, CG; Neiss, A; Poy, F; Romero, FA; Sandy, P; Tang, Y; Tsui, V; Zawadzke, L; Sims, RJ; Audia, JE; Bellon, SF; Magnuson, SR; Albrecht, BK; Cochran, AG J Med Chem 65: 11177-11186 (2022)
- Chamberlain, BT; Rice, JM; Jernigan, III, FE; Sherman, W; Kulkarni, MM; Shechter, S; Allen, BK; Tan, D; Marino, KA; Lin, Z US Patent US11414387 (2022)
- Ruebsam, F; Sun, Z; Ayida, BK; Webber, SE; Zhou, Y; Zhao, Q; Kissinger, CR; Showalter, RE; Shah, AM; Tsan, M; Patel, R; Lebrun, LA; Kamran, R; Sergeeva, MV; Bartkowski, DM; Nolan, TG; Norris, DA; Kirkovsky, L Bioorg Med Chem Lett 18: 5002-5 (2008)
- Biswal, BK; Morisseau, C; Garen, G; Cherney, MM; Garen, C; Niu, C; Hammock, BD; James, MN J Mol Biol 381: 897-912 (2008)
- Blackburn, BK; Lee, A; Baier, M; Kohl, B; Olivero, AG; Matamoros, R; Robarge, KD; McDowell, RS J Med Chem 40: 717-29 (1997)
- Rodriguez, D; Ramesh, C; Henson, LH; Wilmeth, L; Bryant, BK; Kadavakollu, S; Hirsch, R; Montoya, J; Howell, PR; George, JM; Alexander, D; Johnson, DL; Arterburn, JB; Shuster, CB Bioorg Med Chem 19: 5446-53 (2011)
- Sobarzo-Sánchez, EM; Arbaoui, J; Protais, P; Cassels, BK J Nat Prod 63: 480-4 (2000)
- Chan, BK; Hanan, EJ; Bowman, KK; Bryan, MC; Burdick, D; Chan, E; Chen, Y; Clausen, S; Dela Vega, T; Dotson, J; Eigenbrot, C; Elliott, RL; Heald, RA; Jackson, PS; Knight, JD; La, H; Lainchbury, MD; Malek, S; Purkey, HE; Schaefer, G; Schmidt, S; Seward, EM; Sideris, S; Shao, L; Wang, S; Yeap, SK; Yen, I; Yu, C; Heffron, TP J Med Chem 59: 9080-9093 (2016)
- Gray, NM; Cheng, BK; Mick, SJ; Lair, CM; Contreras, PC J Med Chem 32: 1242-8 (1989)
- Colletti, SL; Frie, JL; Dixon, EC; Singh, SB; Choi, BK; Scapin, G; Fitzgerald, CE; Kumar, S; Nichols, EA; O'Keefe, SJ; O'Neill, EA; Porter, G; Samuel, K; Schmatz, DM; Schwartz, CD; Shoop, WL; Thompson, CM; Thompson, JE; Wang, R; Woods, A; Zaller, DM; Doherty, JB J Med Chem 46: 349-52 (2003)
- Du, J; Chun, BK; Mosley, RT; Bansal, S; Bao, H; Espiritu, C; Lam, AM; Murakami, E; Niu, C; Micolochick Steuer, HM; Furman, PA; Sofia, MJ J Med Chem 57: 1826-35 (2014)
- Gao, DA; Xiong, Z; Heim-Riether, A; Amodeo, L; August, EM; Cao, X; Ciccarelli, L; Collins, BK; Harrington, K; Haverty, K; Hill-Drzewi, M; Li, X; Liang, S; Margarit, SM; Moss, N; Nagaraja, N; Proudfoot, J; Roman, R; Schlyer, S; Keenan, LS; Taylor, S; Wellenzohn, B; Wiedenmayer, D; Li, J; Farrow, NA Bioorg Med Chem Lett 20: 5039-43 (2010)
- Corkey BK
- Babu, BS; Kumar, SR; Cho-Hua, LC; Igor, M; Duane, BK; Qinhua, HP; Mohammad, A WIPO WO2023287730 (2023)
- Carrillo García, C; Becker, C; Forster, M; Lohmann, S; Freitag, P; Laufer, S; Sievers, S; Fleischmann, BK; Hesse, M; Schade, D J Med Chem 65: 1505-1524 (2022)
- Attwood, MR; Conway, EA; Dunsdon, RM; Greening, JR; Handa, BK; Jones, PS; Jordan, SC; Keech, E; Wilson, FX Bioorg Med Chem Lett 7: 429-432 (1997)
- Harrington BK
- Ann, J; Ki, Y; Yoon, S; Kim, MS; Lee, JU; Kim, C; Lee, S; Jung, A; Baek, J; Hong, S; Choi, S; Pearce, LV; Esch, TE; Turcios, NA; Lewin, NE; Ogunjirin, AE; Herold, BK; McCall, AK; Blumberg, PM; Lee, J Bioorg Med Chem 24: 1231-40 (2016)
- Imbriglio, JE; Shen, DM; Liang, R; Marby, K; You, M; Youm, HW; Feng, Z; London, C; Xiong, Y; Tata, J; Verras, A; Garcia-Calvo, M; Song, X; Addona, GH; McLaren, DG; He, T; Murphy, B; Metzger, DE; Salituro, G; Deckman, D; Chen, Q; Jin, X; Stout, SJ; Wang, SP; Wilsie, L; Palyha, O; Han, S; Hubbard, BK; Previs, SF; Pinto, S; Taggart, A J Med Chem 58: 9345-53 (2015)
- Green, KD; Biswas, T; Chang, C; Wu, R; Chen, W; Janes, BK; Chalupska, D; Gornicki, P; Hanna, PC; Tsodikov, OV; Joachimiak, A; Garneau-Tsodikova, S Biochemistry 54: 3197-206 (2015)
- Kim, HJ; Jang, BK; Park, JH; Choi, JW; Park, SJ; Byeon, SR; Pae, AN; Lee, YS; Cheong, E; Park, KD Eur J Med Chem 185: (2020)
- Tiwari, AD; Guan, Y; Grabowski, DR; Maciejewski, JP; Jha, BK; Phillips, JG Bioorg Med Chem 39: (2021)
- Burkholder, TP; Kudlacz, EM; Maynard, GD; Liu, XG; Le, TB; Webster, ME; Horgan, SW; Wenstrup, DL; Freund, DW; Boyer, F; Bratton, L; Gross, RS; Knippenberg, RW; Logan, DE; Jones, BK; Chen, TM; Geary, JL; Correll, MA; Poole, JC; Mandagere, AK Bioorg Med Chem Lett 7: 2531-2536 (1997)
- Cardellina, JH; Roxas-Duncan, VI; Montgomery, V; Eccard, V; Campbell, Y; Hu, X; Khavrutskii, I; Tawa, GJ; Wallqvist, A; Gloer, JB; Phatak, NL; Höller, U; Soman, AG; Joshi, BK; Hein, SM; Wicklow, DT; Smith, LA ACS Med Chem Lett 3: 387-391 (2012)
- Heffeter, P; Pongratz, M; Steiner, E; Chiba, P; Jakupec, MA; Elbling, L; Marian, B; Körner, W; Sevelda, F; Micksche, M; Keppler, BK; Berger, W J Pharmacol Exp Ther 312: 281-9 (2004)
- Kim, BK; Kim, JH; Kim, NR; Lee, WG; Lee, SD; Yun, SH; Jeon, ES; Kim, YC Bioorg Med Chem Lett 22: 6952-6 (2012)
- Saccomano, NA; Vinick, FJ; Koe, BK; Nielsen, JA; Whalen, WM; Meltz, M; Phillips, D; Thadieo, PF; Jung, S; Chapin, DS J Med Chem 34: 291-8 (1991)
- O'Brien, PM; Sliskovic, DR; Bernabei, A; Hurley, T; Anderson, MK; Bousley, RF; Krause, BK; Stanfield, RL Bioorg Med Chem Lett 5: 295-300 (1995)
- Pallavi, P; Pretze, M; Caballero, J; Li, Y; Hofmann, BB; Stamellou, E; Klotz, S; Wängler, C; Wängler, B; Loesel, R; Roth, S; Theisinger, B; Moerz, H; Binzen, U; Greffrath, W; Treede, RD; Harmsen, MC; Krämer, BK; Hafner, M; Yard, BA; Kälsch, AI J Med Chem 61: 3126-3137 (2018)
- Al-Awadhi, FH; Law, BK; Paul, VJ; Luesch, H J Nat Prod 80: 2969-2986 (2017)
- Wolkenberg, SE; Zhao, Z; Wisnoski, DD; Leister, WH; O'Brien, J; Lemaire, W; Williams, DL; Jacobson, MA; Sur, C; Kinney, GG; Pettibone, DJ; Tiller, PR; Smith, S; Gibson, C; Ma, BK; Polsky-Fisher, SL; Lindsley, CW; Hartman, GD Bioorg Med Chem Lett 19: 1492-5 (2009)
- Meltzer, PC; Butler, D; Deschamps, JR; Madras, BK J Med Chem 49: 1420-32 (2006)
- Cee, VJ; Chavez, F; Herberich, B; Lanman, BA; Pettus, LH; Reed, AB; Wu, B; Wurz, RP; Andrews, KL; Chen, J; Hickman, D; Laszlo, J; Lee, MR; Guerrero, N; Mattson, BK; Nguyen, Y; Mohr, C; Rex, K; Sastri, CE; Wang, P; Wu, Q; Wu, T; Xu, Y; Zhou, Y; Winston, JT; Lipford, JR; Tasker, AS; Wang, HL ACS Med Chem Lett 7: 408-12 (2016)
- Aldrich, LN; Burdette, JE; Carcache de Blanco, E; Coss, CC; Eustaquio, AS; Fuchs, JR; Kinghorn, AD; MacFarlane, A; Mize, BK; Oberlies, NH; Orjala, J; Pearce, CJ; Phelps, MA; Rakotondraibe, LH; Ren, Y; Soejarto, DD; Stockwell, BR; Yalowich, JC; Zhang, X J Nat Prod 85: 702-719 (2022)
- Hobson, AD; Judge, RA; Aguirre, AL; Brown, BS; Cui, Y; Ding, P; Dominguez, E; DiGiammarino, E; Egan, DA; Freiberg, GM; Gopalakrishnan, SM; Harris, CM; Honore, MP; Kage, KL; Kapecki, NJ; Ling, C; Ma, J; Mack, H; Mamo, M; Maurus, S; McRae, B; Moore, NS; Mueller, BK; Mueller, R; Namovic, MT; Patel, K; Pratt, SD; Putman, CB; Queeney, KL; Sarris, KK; Schaffter, LM; Stoll, V; Vasudevan, A; Wang, L; Wang, L; Wirthl, W; Yach, K J Med Chem 61: 11074-11100 (2018)
- Shaik, JB; Palaka, BK; Penumala, M; Eadlapalli, S; Darla Mark, M; Ampasala, DR; Vadde, R; Amooru Gangaiah, D Chem Biol Drug Des 88: 43-53 (2016)
- Stocks, PA; Raynes, KJ; Bray, PG; Park, BK; O'Neill, PM; Ward, SA J Med Chem 45: 4975-83 (2002)
- Sanchez-Martinez, C; Shih, C; Zhu, G; Li, T; Brooks, HB; Patel, BK; Schultz, RM; DeHahn, TB; Spencer, CD; Watkins, SA; Ogg, CA; Considine, E; Dempsey, JA; Zhang, F Bioorg Med Chem Lett 13: 3841-6 (2003)
- Prabhala BK
- Davoren, JE; Nason, D; Coe, J; Dlugolenski, K; Helal, C; Harris, AR; LaChapelle, E; Liang, S; Liu, Y; O'Connor, R; Orozco, CC; Rai, BK; Salafia, M; Samas, B; Xu, W; Kozak, R; Gray, D J Med Chem 61: 11384-11397 (2018)
- Ruan BK
- YOON, SH; JOO, HW; SEO, BK; LEE, EJ; JUNG, JY; YOON, SY; CHO, WY US Patent US20230322706 (2023)
- Ghosh, KS; Debnath, J; Dutta, P; Sahoo, BK; Dasgupta, S Bioorg Med Chem 16: 2819-28 (2008)
- Sarma, BK; Liu, X; Kodadek, T Bioorg Med Chem 24: 3953-3963 (2016)
- Goodman, KB; Bury, MJ; Cheung, M; Cichy-Knight, MA; Dowdell, SE; Dunn, AK; Lee, D; Lieby, JA; Moore, ML; Scherzer, DA; Sha, D; Suarez, DP; Murphy, DJ; Harpel, MR; Manas, ES; McNulty, DE; Annan, RS; Matico, RE; Schwartz, BK; Trill, JJ; Sweitzer, TD; Wang, DY; Keller, PM; Krawiec, JA; Jaye, MC Bioorg Med Chem Lett 19: 27-30 (2008)
- Sharma, S; Sharma, BK; Sharma, SK; Singh, P; Prabhakar, YS Eur J Med Chem 44: 1377-82 (2009)
- Parsons, WH; Rutland, NT; Crainic, JA; Cardozo, JM; Chow, AS; Andrews, CL; Sheehan, BK Bioorg Med Chem Lett 49: (2021)
- McGovern, SL; Helfand, BT; Feng, B; Shoichet, BK J Med Chem 46: 4265-72 (2003)
- Kumar Singh, A; Rajendran, V; Singh, S; Kumar, P; Kumar, Y; Singh, A; Miller, W; Potemkin, V; Poonam, na; Grishina, M; Gupta, N; Kempaiah, P; Durvasula, R; Singh, BK; Dunn, BM; Rathi, B Bioorg Med Chem 26: 3837-3844 (2018)
- Sinha, BK; Cysyk, RL; Millar, DB; Chignell, CF J Med Chem 19: 994-8 (1976)
- Lahm, GP; Stevenson, TM; Selby, TP; Freudenberger, JH; Cordova, D; Flexner, L; Bellin, CA; Dubas, CM; Smith, BK; Hughes, KA; Hollingshaus, JG; Clark, CE; Benner, EA Bioorg Med Chem Lett 17: 6274-9 (2007)
- von Geldern, TW; Tasker, AS; Sorensen, BK; Winn, M; Szczepankiewicz, BG; Dixon, DB; Chiou, WJ; Wang, L; Wessale, JL; Adler, A; Marsh, KC; Nguyen, B; Opgenorth, TJ J Med Chem 42: 3668-78 (1999)
- Srivastava, BK; Joharapurkar, A; Raval, S; Patel, JZ; Soni, R; Raval, P; Gite, A; Goswami, A; Sadhwani, N; Gandhi, N; Patel, H; Mishra, B; Solanki, M; Pandey, B; Jain, MR; Patel, PR J Med Chem 50: 5951-66 (2007)
- Lowe, MA; Cardenas, A; Valentin, JP; Zhu, Z; Abendroth, J; Castro, JL; Class, R; Delaunois, A; Fleurance, R; Gerets, H; Gryshkova, V; King, L; Lorimer, DD; MacCoss, M; Rowley, JH; Rosseels, ML; Royer, L; Taylor, RD; Wong, M; Zaccheo, O; Chavan, VP; Ghule, GA; Tapkir, BK; Burrows, JN; Duffey, M; Rottmann, M; Wittlin, S; Angulo-Barturen, I; Jiménez-Díaz, MB; Striepen, J; Fairhurst, KJ; Yeo, T; Fidock, DA; Cowman, AF; Favuzza, P; Crespo-Fernandez, B; Gamo, FJ; Goldberg, DE; Soldati-Favre, D; Laleu, B; de Haro, T J Med Chem 65: 14121-14143 (2022)
- Heightman, TD; Gaster, LM; Pardoe, SL; Pilleux, JP; Hadley, MS; Middlemiss, DN; Price, GW; Roberts, C; Scott, CM; Watson, JM; Gordon, LJ; Holland, VA; Powles, J; Riley, GJ; Stean, TO; Trail, BK; Upton, N; Austin, NE; Ayrton, AD; Coleman, T; Cutler, L Bioorg Med Chem Lett 15: 4370-4 (2005)
- Dixit, M; Tripathi, BK; Tamrakar, AK; Srivastava, AK; Kumar, B; Goel, A Bioorg Med Chem 15: 727-34 (2006)
- Augelli-Szafran, CE; Blankley, CJ; Roth, BD; Trivedi, BK; Bousley, RF; Essenburg, AD; Hamelehle, KL; Krause, BR; Stanfield, RL J Med Chem 36: 2943-9 (1993)
- Abdelfadiel, EI; Gunta, R; Villuri, BK; Afosah, DK; Sankaranarayanan, NV; Desai, UR J Med Chem 66: 4503-4531 (2023)
- Lang, L; Jagoda, E; Schmall, B; Vuong, BK; Adams, HR; Nelson, DL; Carson, RE; Eckelman, WC J Med Chem 42: 1576-86 (1999)
- CARZANIGA, L; RANCATI, F; RIZZI, A; KARAWAJCZYK, A; WOLEK, BK; MULLINS, TM US Patent US20240190847 (2024)
- Wagner, FF; Lundh, M; Kaya, T; McCarren, P; Zhang, YL; Chattopadhyay, S; Gale, JP; Galbo, T; Fisher, SL; Meier, BC; Vetere, A; Richardson, S; Morgan, NG; Christensen, DP; Gilbert, TJ; Hooker, JM; Leroy, M; Walpita, D; Mandrup-Poulsen, T; Wagner, BK; Holson, EB ACS Chem Biol 11: 363-74 (2016)
- Dyckman, AJ; Dodd, DS; Mussari, CP; Sherwood, TC; Whiteley, BK; Gilmore, JL; Kumar, SR; Pasunoori, L; Srinivas, PV; Duraisamy, SK; Hegde, S; Anumula, RK US Patent US11053244 (2021)
- Bell, IM; Bednar, RA; Corcoran, HA; Fay, JF; Gallicchio, SN; Johnston, VK; Hershey, JC; Miller-Stein, CM; Moore, EL; Mosser, SD; Roller, SA; Salvatore, CA; Theberge, CR; Wong, BK; Blair Zartman, C; Kane, SA; Williams, TM; Graham, SL; Vacca, JP Bioorg Med Chem Lett 19: 4740-2 (2009)
- Yap, BK; Leung, EW; Yagi, H; Galea, CA; Chhabra, S; Chalmers, DK; Nicholson, SE; Thompson, PE; Norton, RS J Med Chem 57: 7006-15 (2014)
- Mohammadi, M; McMahon, G; Sun, L; Tang, C; Hirth, P; Yeh, BK; Hubbard, SR; Schlessinger, J Science 276: 955-60 (1997)
- Yeung, BK; Zou, B; Rottmann, M; Lakshminarayana, SB; Ang, SH; Leong, SY; Tan, J; Wong, J; Keller-Maerki, S; Fischli, C; Goh, A; Schmitt, EK; Krastel, P; Francotte, E; Kuhen, K; Plouffe, D; Henson, K; Wagner, T; Winzeler, EA; Petersen, F; Brun, R; Dartois, V; Diagana, TT; Keller, TH J Med Chem 53: 5155-64 (2010)
- Sağlık, BN; Osmaniye, D; Çevik, UA; Levent, S; Çavuşoğlu, BK; Büyükemir, O; Nezir, D; Karaduman, AB; Özkay, Y; Koparal, AS; Beydemir, Ş; Kaplancıklı, ZA Eur J Med Chem 198: (2020)
- Kale, RR; Kale, MG; Waterson, D; Raichurkar, A; Hameed, SP; Manjunatha, MR; Kishore Reddy, BK; Malolanarasimhan, K; Shinde, V; Koushik, K; Jena, LK; Menasinakai, S; Humnabadkar, V; Madhavapeddi, P; Basavarajappa, H; Sharma, S; Nandishaiah, R; Mahesh Kumar, KN; Ganguly, S; Ahuja, V; Gaonkar, S; Naveen Kumar, CN; Ogg, D; Boriack-Sjodin, PA; Sambandamurthy, VK; de Sousa, SM; Ghorpade, SR Bioorg Med Chem Lett 24: 870-9 (2014)
- STOKES, S; BEDFORD, S; CHEN, null; DAVIDSON, JE; DAVIES, N; GRAHAM, CJ; MCKENNA, SM; MEISSNER, JW; MURRAY, JB; PARSONS, RJ; RAY, S; SANDERS, E; WALMSLEY, CL; BROUGH, PA; KOTSCHY, A; PROSZENYÁK, null; SINAI, null; BÁLINT, BK; CSÉKEI, M; ZWILLINGER, M; GARAMVÖLGYI, R; SIPOS, S; KUN, V; SZABÓ, Z; CHANRION, M; ROCCHETTI, F; COLLAND, F; MARAGNO, AL; BRESSON, L US Patent US20240150293 (2024)
- ChEMBL_644658 (CHEMBL1211637) Displacement of [3H]Lys-desArg9-BK from human bradykinin B1 receptor
- ChEMBL_1861204 (CHEMBL4362060) Displacement of [3H]BK from Bradykinin B2 Receptor in guinea pig ileum by liquid scintillation counter
- ChEMBL_216334 (CHEMBL818748) Inhibition of specific binding of [3H]BK to bradykinin B2 receptors of guinea pig ileum (GPI)
- ChEMBL_216335 (CHEMBL818749) Inhibition of specific binding of [3H]BK to bradykinin B2 receptors of guinea pig ileum (GPI)
- ChEMBL_40267 (CHEMBL656503) Concentration required to inhibit specific binding of [3H]-BK(0.06 nM) to the bradykinin receptor B2
- ChEMBL_40430 (CHEMBL876526) Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells
- ChEMBL_662792 (CHEMBL1252531) Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells
- ChEMBL_40291 (CHEMBL653423) Inhibition of [3H]BK (1.0 nM) binding to the human bradykinin receptor B2, expressed in CHO cells
- ChEMBL_644657 (CHEMBL1211636) Antagonist activity at human bradykinin B1 receptor assessed as inhibition of Lys-desArg9-BK-induced calcium flux
- ChEMBL_40274 (CHEMBL656509) Inhibition of the specific binding of [3H]BK to Bradykinin receptor B2 in guinea pig ileum membrane preparations
- ChEMBL_40294 (CHEMBL884128) Inhibition of the specific binding of [3H]BK to human recombinant Bradykinin receptor B2 expressed in CHO cells.
- ChEBML_40107 Binding affinity towards human bradykinin B2 receptor (membranes from Cos-7 cells expressing human B2 receptor) using [3H]-BK as radioligand
- ChEMBL_40443 (CHEMBL652382) Inhibition of binding of [3H]BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes
- ChEMBL_1560590 (CHEMBL3777322) Displacement of [3H]BK from cloned human B2 bradykinin receptor expressed in CHO cells after 2 hrs by liquid scintillation counter
- ChEMBL_40269 (CHEMBL856079) Concentration required to inhibit specific binding of [3H]-BK(0.06 nM) to the bradykinin receptor B2 in guinea pig ileum membrane
- ChEMBL_40292 (CHEMBL653424) In vitro inhibitory activity towards human bradykinin receptor B2 expressed in CHO cells using [3H]BK (1.0 nM) as a radioligand
- ChEMBL_40293 (CHEMBL653425) Inhibition of specific binding of [3H]BK at 1 nM to human bradykinin receptor B2 expressed in CHO cells by 50%.
- ChEMBL_40444 (CHEMBL652383) Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes
- ChEMBL_40276 (CHEMBL653409) Binding affinity towards bradykinin receptor B2 using [3H]bradykinin
- ChEBML_40436 In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay
- ChEMBL_40262 (CHEMBL653278) In vitro for inhibition of specific binding of [3H]BK (0.06 nM) to bradykinin receptor B2 in guinea pig ileum membrane preparations.
- ChEMBL_40272 (CHEMBL656507) In vitro inhibitory activity towards bradykinin receptor B2 using [3H]BK (0.06 nM) as a radioligand in guinea pig ileum membrane preparation
- ChEMBL_40273 (CHEMBL656508) Inhibition of specific binding of [3H]BK at 0.06 nM to bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%.
- ChEMBL_1559755 (CHEMBL3779535) Displacement of [3H]bradykinin from human recombinant B2 bradykinin receptor
- ChEMBL_40263 (CHEMBL653279) Concentration required to inhibit specific binding of [ 3H]BK (0.06 nM) to Bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%
- ChEMBL_40265 (CHEMBL656501) Concentration required to inhibit specific binding of [ 3H]BK (0.06 nM) to Bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%.
- ChEMBL_40436 (CHEMBL883337) In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay
- ChEBML_40423 Binding affinity against human bradykinin receptor B2 using [3H]bradykinin as radioligand
- ChEMBL_40264 (CHEMBL653280) Concentration required to inhibit specific binding of [3H]BK at 0.06 nM to Bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%.
- ChEMBL_40286 (CHEMBL653418) Inhibition specific binding of [3H]BK (1.0 nM) to human Bradykinin receptor B2 which was expressed in CHO (Chinese hamster ovary) cells by 50%.
- ChEMBL_40423 (CHEMBL652638) Binding affinity against human bradykinin receptor B2 using [3H]bradykinin as radioligand
- ChEMBL_40289 (CHEMBL653421) Concentration required to inhibit specific binding of [3H]BK (1.2 nM) to A-431 cells (human epidermoid carcinoma) which express Bradykinin receptor B2 by 50%.
- ChEMBL_429986 (CHEMBL917716) Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO cells
- ChEMBL_40288 (CHEMBL653420) Concentration required to inhibit specific binding of [3H]BK at 1.2 nM to A-431 cells (human epidermoid carcinoma) which express Bradykinin receptor B2 by 50%.
- ChEBML_40275 Binding affinity against [3H]bradykinin binding to bradykinin receptor B2 in guinea pig ileal membrane
- ChEMBL_425212 (CHEMBL855158) Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells
- ChEMBL_643606 (CHEMBL1212470) Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells
- ChEMBL_806223 (CHEMBL1958764) Displacement of [3H][desArg9]Lys-Bradykinin from human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_40426 (CHEMBL652641) Binding affinity towards human cloned Bradykinin receptor B2 expressed in CHO cells by [3H]bradykinin displacement.
- ChEMBL_40441 (CHEMBL652380) Binding affinity against rat Bradykinin receptor B2 expressed in CHO cells using [3H]-bradykinin as radioligand
- ChEMBL_40442 (CHEMBL652381) Binding affinity against rat bradykinin B2 receptors expressed in CHO cells using [3H]bradykinin as radioligand
- ChEMBL_40130 (CHEMBL658496) Binding affinity against human cloned Bradykinin receptor B1 expressed in CHO cells using [3H]-bradykinin as radioligand
- ChEMBL_40422 (CHEMBL652637) Binding affinity against human cloned Bradykinin receptor B2 expressed in CHO cells using [3H]-bradykinin as radioligand
- ChEMBL_965476 (CHEMBL2395280) Displacement of [3H]Bradykinin from human recombinant bradykinin B2 receptor expressed in CHEM1 cells after 60 mins
- Binding Assay Binding assay using Bradykinin-1 receptor.
- ChEBML_40424 Binding affinity towards human bradykinin receptor B2
- ChEMBL_333385 (CHEMBL859129) Binding affinity to bradykinin B2 receptor
- ChEMBL_39982 (CHEMBL653556) Inhibition of human bradykinin B1 receptor
- ChEMBL_423710 (CHEMBL913022) Inhibition of human bradykinin B2 receptor
- ChEMBL_535289 (CHEMBL982614) Inhibition of human bradykinin B1 receptor
- ChEMBL_662793 (CHEMBL1252532) Inhibition of rabbit bradykinin B1 receptor
- ChEMBL_662794 (CHEMBL1252533) Inhibition of rat bradykinin B1 receptor
- ChEMBL_879298 (CHEMBL2208696) Antagonist activity at Bradykinin B1 receptor
- ChEMBL_497315 (CHEMBL995877) Displacement of [3H]Lys-bradykinin from human recombinant bradykinin B1 receptor expressed in CHO cells by scintillation counting
- ChEBML_40429 In vitro binding affinity against Bradykinin receptor B2
- ChEMBL_2297250 Binding affinity to B2 bradykinin receptor (unknown origin)
- ChEMBL_302466 (CHEMBL826332) Binding affinity against Human bradykinin receptor B1
- ChEMBL_423681 (CHEMBL853755) Binding affinity to rat bradykinin B1 receptor
- ChEMBL_423682 (CHEMBL853760) Binding affinity to rabbit bradykinin B1 receptor
- ChEMBL_429982 (CHEMBL917712) Binding affinity to rabbit bradykinin B1 receptor
- ChEMBL_434373 (CHEMBL919421) Antagonist activity at rat bradykinin B1 receptor
- ChEMBL_434374 (CHEMBL919418) Antagonist activity at rabbit bradykinin B1 receptor
- ChEMBL_443978 (CHEMBL893140) Binding affinity at human bradykinin B1 receptor
- ChEMBL_487377 (CHEMBL1021904) Binding affinity to human bradykinin B1 receptor
- ChEMBL_535281 (CHEMBL982606) Antagonist activity at rat bradykinin B1 receptor
- ChEMBL_662796 (CHEMBL1252535) Binding affinity to human bradykinin B1 receptor
- ChEMBL_662797 (CHEMBL1252536) Antagonist activity at dog bradykinin B1 receptor
- ChEMBL_662798 (CHEMBL1252537) Binding affinity to rat bradykinin B1 receptor
- ChEMBL_662799 (CHEMBL1252538) Binding affinity to mouse bradykinin B1 receptor
- ChEMBL_662800 (CHEMBL1252539) Binding affinity to monkey bradykinin B1 receptor
- ChEMBL_748611 (CHEMBL1780464) Antagonist activity at human bradykinin B1 receptor
- ChEMBL_796433 (CHEMBL1937748) Binding affinity to human B1 bradykinin receptor
- ChEMBL_814368 (CHEMBL2019577) Binding affinity to human bradykinin B1 receptor
- ChEMBL_814371 (CHEMBL2019580) Antagonist activity at rat bradykinin B1 receptor
- ChEMBL_610511 (CHEMBL1064982) Antagonist activity at bradykinin B1 receptor in human IMR90 cells assessed as inhibition of des-Arg-bradykinin-mediated calcium mobilization
- ChEMBL_612385 (CHEMBL1066271) Antagonist activity at bradykinin B1 receptor in human IMR90 cells assessed as inhibition of des-Arg-bradykinin-mediated calcium mobilization
- ChEMBL_806221 (CHEMBL1958762) Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting
- ChEMBL_806231 (CHEMBL1958772) Antagonist activity at guinea pig bradykinin B2 receptor in longitudinal smooth muscle assessed as inhibition of bradykinin-induced contractile responses
- ChEMBL_1651749 (CHEMBL4001004) Antagonist activity against bradykinin B1 receptor in human HeLa cells assessed as inhibition of Des-Arg9-BK-induced increase in intracellular Ca2+ level by Fluro-4 direct calcium dye based fluorescence assay
- ChEMBL_555877 (CHEMBL955851) Inhibition of HCV BK NS5B polymerase
- ChEMBL_523549 (CHEMBL1001563) Antagonist activity at human recombinant bradykinin 2 receptor expressed in CHO cells assessed as inhibition of bradykinin-induced intracellular calcium mobilization
- Calcium Mobilization Assay Calcium mobilization assay using Bradykinin-1 receptor.
- ChEMBL_2078239 (CHEMBL4734030) Agonist activity at bradykinin B2 receptor (unknown origin)
- ChEMBL_423683 (CHEMBL853765) Binding affinity to rhesus monkey bradykinin B1 receptor
- ChEMBL_658534 (CHEMBL1247736) Binding affinity to human recombinant bradykinin B1 receptor
- ChEMBL_658535 (CHEMBL1247737) Binding affinity to human recombinant bradykinin B2 receptor
- ChEMBL_1633556 (CHEMBL3876348) Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells measured after 60 mins by scintillation counting method
- ChEMBL_1861205 (CHEMBL4362061) Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells incubated for 2 hrs by liquid scintillation counter
- ChEMBL_806222 (CHEMBL1958763) Antagonist activity at human bradykinin B2 receptor expressed in dhfr-deficient CHO cells assessed as inhibition of bradykinin-induced inositol monophosphate accumulation preincubated for 15 mins prior bradykinin induction measured after 40 mins by liquid scintillation spectrometry
- ChEMBL_305717 (CHEMBL829390) Inhibition of Bradykinin receptor B1 expressed in HEK293 cells
- ChEMBL_572632 (CHEMBL1026056) Displacement of [3H]bradykinin from wild-type B2 receptor
- ChEMBL_572638 (CHEMBL1026062) Displacement of [3H]bradykinin from guinea pig B2 receptor
- ChEMBL_351511 (CHEMBL870070) Inhibition of HCV 1b BK NS5B RNA polymerase
- ChEBML_40431 Tested for binding affinity against human IMR-90 Bradykinin receptor B2
- ChEMBL_2023082 (CHEMBL4676895) Displacement of [3H]nicotine from B2 bradykinin receptor (unknown origin)
- ChEMBL_302814 (CHEMBL839504) Binding affinity for Bradykinin receptor B2 expressed in COS7 cells
- ChEMBL_302842 (CHEMBL827887) Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells
- ChEMBL_321573 (CHEMBL881788) Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived
- ChEMBL_40138 (CHEMBL876936) Compound was tested for inhibition against rat Bradykinin receptor B1
- ChEMBL_429983 (CHEMBL917713) Activity at rabbit bradykinin B1 receptor assessed by FLIPR assay
- ChEMBL_443979 (CHEMBL893141) Antagonist activity in human bradykinin B1 receptor by FLIPR method
- ChEMBL_463210 (CHEMBL947207) Binding affinity to human bradykinin B1 receptor by FLIPR assay
- ChEMBL_2059792 (CHEMBL4714793) Inhibition of BK (unknown origin) by patch clamp analysis
- ChEMBL_337081 (CHEMBL864366) Inhibitory activity against HCV 1b BK NS5B RNA polymerase
- ChEBML_40277 In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum.
- ChEBML_40427 Binding affinity against human IMR 90 fetal lung fibroblast bradykinin receptor B2
- ChEMBL_1561510 (CHEMBL3776147) Inhibition of recombinant human bradykinin receptor 2 expressed in CHO cells
- ChEMBL_321543 (CHEMBL881481) Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate
- ChEMBL_333384 (CHEMBL859128) Binding affinity to human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_40431 (CHEMBL652645) Tested for binding affinity against human IMR-90 Bradykinin receptor B2
- ChEMBL_463209 (CHEMBL947206) Binding affinity to human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463211 (CHEMBL947208) Binding affinity to rat bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463212 (CHEMBL947209) Binding affinity to monkey bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463213 (CHEMBL947210) Binding affinity to rabbit bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463214 (CHEMBL947211) Binding affinity to dog bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463823 (CHEMBL948902) Binding affinity to human bradykinin B1 receptor expressed in rat CNS
- ChEMBL_796432 (CHEMBL1937747) Antagonist activity at rabbit B1 bradykinin receptor expressed in CHO cells
- ChEMBL_964329 (CHEMBL2395591) Binding affinity to human bradykinin B2 receptor by radioligand displacement assay
- ChEMBL_966197 (CHEMBL2395702) Binding affinity to human bradykinin B2 receptor by radioligand displacement assay
- ChEMBL_337076 (CHEMBL864354) Inhibitory activity against HCV 1b BK NS5B deltaC55 RNA polymerase
- ChEMBL_337080 (CHEMBL864365) Inhibitory activity against HCV 1b BK NS5B deltaC21 RNA polymerase
- ChEMBL_337082 (CHEMBL864369) Inhibitory activity against HCV 2a BK NS5B deltaC21 RNA polymerase
- ChEMBL_337083 (CHEMBL864371) Inhibitory activity against HCV 3a BK NS5B deltaC21 RNA polymerase
- ChEMBL_386030 (CHEMBL870300) Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase
- ChEMBL_400597 (CHEMBL853550) Inhibitory activity against HCV 1b BK NS5B deltaC21 RNA polymerase
- ChEBML_40106 Binding affinity against human IMR90 fetal lung fibroblast bradykinin B2 receptor was evaluated
- ChEBML_40141 Compound was evaluated for competitive [3H]bradykinin binding to guinea pig ileum homogenates
- ChEMBL_302733 (CHEMBL838681) Binding affinity of the [35S]- radiolabeled compound to dog Bradykinin receptor B1
- ChEMBL_302734 (CHEMBL876354) Binding affinity of the [35S]- radiolabeled compound to rat Bradykinin receptor B1
- ChEMBL_302791 (CHEMBL839475) Binding affinity of the [35S]- radiolabeled compound to rabbit Bradykinin receptor B1
- ChEMBL_40139 (CHEMBL656133) Antagonistic activity against Bradykinin receptor B1 of rat ileum longitudinal smooth muscle.
- ChEMBL_40427 (CHEMBL652642) Binding affinity against human IMR 90 fetal lung fibroblast bradykinin receptor B2
- ChEMBL_40437 (CHEMBL652376) Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscle
- ChEMBL_337078 (CHEMBL864362) Inhibition of replication of HCV 1b BK RNA in Huh7 cells
- ChEMBL_302897 (CHEMBL830212) Binding affinity of the [35S]- radiolabeled compound to rhesus monkey Bradykinin receptor B1
- ChEMBL_302988 (CHEMBL830227) Inhibitory constant against human Bradykinin receptor B1 expressed in chinese hamster ovary cells
- ChEMBL_39981 (CHEMBL653555) Compound was tested for inhibition against human bradykinin B1 receptor using FLIPR assay
- ChEMBL_40135 (CHEMBL656787) In vitro Bradykinin receptor B1 antagonist activity in functional tissue within rabbit aorta
- ChEMBL_40137 (CHEMBL656789) Compound was tested for inhibition against rat Bradykinin receptor B1 using FLIPR assay
- ChEMBL_40279 (CHEMBL653412) Antagonistic activity against Bradykinin receptor B2 of guinea pig ileum longitudinal smooth muscle.
- ChEMBL_40283 (CHEMBL653415) Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (WI38 fibroblasts)
- ChEMBL_40445 (CHEMBL652384) In vitro Bradykinin receptor B2 antagonist activity by using rat uterus functional assay
- ChEMBL_434360 (CHEMBL919419) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells
- ChEMBL_510232 (CHEMBL1005610) Antagonist activity at human bradykinin B1 receptor expressed in CHO-D-/aequorin cells
- ChEMBL_572637 (CHEMBL1026061) Displacement of [3H]bradykinin from human recombinant B2 receptor expressed in HEK293 cells
- ChEBML_70880 Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI)
- ChEMBL_337088 (CHEMBL862549) Inhibitory activity against HCV 1b BK NS5B deltaC55-R158M mutant RNA polymerase
- ChEMBL_337089 (CHEMBL862550) Inhibitory activity against HCV 1b BK NS5B deltaC55-R158K mutant RNA polymerase
- ChEBML_40297 Compound was assayed for binding against the human Bradykinin receptor B2 expressed in CHO cells
- ChEMBL_40271 (CHEMBL656506) In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig pulmonary artery assay.
- ChEMBL_40285 (CHEMBL653417) Antagonism of the [Ca2+] efflux actions of the human Bradykinin receptor B2 (WI38 fibroblasts)
- ChEMBL_425211 (CHEMBL855157) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells
- ChEMBL_337085 (CHEMBL854142) Inhibitory activity against HCV 1b BK NS5B deltaC55 RNA polymerase involving magnesium chelation
- ChEMBL_337086 (CHEMBL862541) Inhibitory activity against HCV 1b BK NS5B deltaC55 RNA polymerase involving manganese chelation
- ChEMBL_70880 (CHEMBL684538) Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI)
- ChEBML_1711424 Displacement of [3H]bradykinin from human recombinant B2 receptor after 60 mins by scintillation counting analysis
- ChEMBL_40270 (CHEMBL656505) In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig ileal membrane receptor assay.
- ChEMBL_40282 (CHEMBL653414) Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (SK-N-SH neuroblastoma)
- ChEMBL_40428 (CHEMBL652643) Ability to bind to human cloned B2 receptor in competition binding experiments with [3H]- bradykinin
- ChEMBL_425214 (CHEMBL855160) Antagonistic activity at rat bradykinin B1 receptor assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_429981 (CHEMBL917711) Displacement of [3H]des-arg10 kallidin from rat bradykinin B1 receptor expressed in CHO cells
- ChEMBL_446485 (CHEMBL895597) Displacement of [3H]DAKA from human bradykinin B1 receptor in IL1-beta stimulated IMR90 cells
- ChEMBL_40425 (CHEMBL652640) Binding affinity towards human cloned B2 receptor was determined using [3H]BK as radioligand
- ChEMBL_426365 (CHEMBL907989) Inhibition of recombinant HCV 1b BK NS5B polymerase by primer/template-directed transcription assay
- ChEMBL_306532 (CHEMBL827821) Antagonist activity against human Bradykinin receptor B1 was determined in a fluorescence imaging plate reader assay
- ChEMBL_40284 (CHEMBL653416) Antagonism of the [Ca2+] efflux actions of the human Bradykinin receptor B2 (SK-N-SH neuroblastoma)
- ChEMBL_423668 (CHEMBL913433) Displacement of [3H]des-Arg10 Leu9 kallidin from human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_429973 (CHEMBL917703) Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_446484 (CHEMBL895596) Antagonist activity at human bradykinin B1 receptor in IL1-beta stimulated IMR90 cells by FLIPR assay
- ChEMBL_512956 (CHEMBL977292) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells
- ChEMBL_532343 (CHEMBL973266) Induction of BK channel mediated vasorelaxation activity in Kcl-induced contraction in Wistar rat aorta
- ChEMBL_558735 (CHEMBL954137) Activation of BK channel-mediated vasorelaxation activity assessed as reduction in KCl-induced contractile tone
- ChEMBL_40419 (CHEMBL652634) Compound was assayed for binding against the human Bradykinin receptor B2 expressed in CHO cells; 0.2-16
- ChEMBL_40421 (CHEMBL652636) Affinity to human Bradykinin receptor B2 in CHO cell membranes determined by displacement of [3H]-NPC 17731
- ChEMBL_425235 (CHEMBL855800) Antagonistic activity at African green monkey bradykinin B1 receptor assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_572639 (CHEMBL1026063) Antagonist activity at B2 receptor in human HF15 cells assessed as inhibition of bradykinin-induced calcium mobilization
- ChEMBL_662795 (CHEMBL1252534) Antagonist activity at human bradykinin B1 receptor in human MR5 cells assessed as [3H]inositol phosphate accumulation
- ChEMBL_796434 (CHEMBL1937749) Antagonist activity at human B1 bradykinin receptor expressed in CHO cells by aqueorin-based calcium flux assay
- ChEMBL_965477 (CHEMBL2395281) Displacement of [3H](Des-Arg10)-Kallidin from bradykinin B1 receptor in human IMR90 cells after 60 mins
- ChEMBL_1474191 (CHEMBL3424209) Inhibition of HCV genotype 1b BK NS5B expressed in Escherichia coli BL21 (DE3) after 1 hr
- ChEMBL_544428 (CHEMBL1016925) Activation of BK channel in Wistar rat thoracic aorta assessed as relaxation of KCl-induced contraction
- ChEMBL_40438 (CHEMBL652377) Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2
- ChEMBL_512957 (CHEMBL977293) Antagonist activity at human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells by aquerin based assay
- ChEMBL_512960 (CHEMBL977296) Antagonist activity at rabbit bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells by aquerin based assay
- ChEMBL_302253 (CHEMBL830269) Equilibrium dissociation constant of the [35S]- radiolabeled compound against human Bradykinin receptor B1 expressed in CHO membranes was determined
- ChEMBL_425210 (CHEMBL855156) Antagonistic activity at human bradykinin B1 receptor expressed in CHO cells assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_434361 (CHEMBL919420) Antagonist activity at human bradykinin B1 receptor expressed in CHOD cells assessed as effect on DAK-induced calcium flux
- ChEMBL_510231 (CHEMBL1005609) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique
- ChEMBL_535280 (CHEMBL982605) Antagonist activity at Cynomolgus monkey bradykinin B1 receptor expressed in CHO cells assessed as calcium transient by FLIPR assay
- ChEMBL_756380 (CHEMBL1805406) Antagonist activity at human bradykinin B1 receptor expressed in human HEK293 cells assessed as calcium flux by FLIPR assay
- ChEMBL_40103 (CHEMBL659261) Compound was evaluated for inhibitory activity against [3H]BK in the classical guinea pig ileum B2 binding assay
- ChEMBL_303697 (CHEMBL828184) Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_303698 (CHEMBL828185) Binding affinity to human Bradykinin receptor B2 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_425213 (CHEMBL855159) Antagonistic activity at rabbit bradykinin B1 receptor expressed in CHO-D cells assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_492574 (CHEMBL952241) Displacement of [3H]des-arg10leu9kallidin from human bradykinin B1 receptor expressed in CHO cells by Wallac beta-plate scintillation counting
- ChEMBL_1516518 (CHEMBL3619869) Displacement of [3H]BK from B2R in rat brain membranes by liquid scintillation counting in based radioligand competition assay
- ChEMBL_376990 (CHEMBL866684) Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase
- ChEMBL_852821 (CHEMBL2155420) Antagonist activity at human bradykinin B1 receptor expressed in human CHO cells assessed as inhibition of calcium flux by FLIPR assay
- ChEMBL_1516520 (CHEMBL3619871) Displacement of [3H]BK from B2R in guinea pig ileum membranes by liquid scintillation counting in based radioligand competition assay
- ChEMBL_40266 (CHEMBL656502) Concentration required to inhibit specific binding of [3H]-BK(0.06 nM) to the B2 receptor in guinea pig ileum membrane;
- ChEMBL_612393 (CHEMBL1066279) Antagonist activity at bradykinin B1 receptor in human IMR90 cells pretreated with IL1-beta assessed as inhibition of DAKD-induced calcium mobilization
- ChEMBL_797691 (CHEMBL1943911) Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting
- ChEMBL_1516519 (CHEMBL3619870) Displacement of [3H]BK from human B2R expressed in HEK293 cell membranes by liquid scintillation counting in based radioligand competition assay
- ChEMBL_1856690 (CHEMBL4357419) Binding affinity to NT-495 fluorophore reagent labelled human BK beta4 Q124A mutant incubated for 30 mins by microscale thermophoresis assay
- ChEMBL_1856692 (CHEMBL4357421) Binding affinity to NT-495 fluorophore reagent labelled human BK beta4 E104K mutant incubated for 30 mins by microscale thermophoresis assay
- ChEMBL_1550090 (CHEMBL3756036) Inhibition of Pseudomonas aeruginosa pseudolysin pretreated with compound for 1 hr followed by the addition of 1.73 uM bradykinin like substrate by spectrofluorometry
- ChEMBL_423671 (CHEMBL912896) Activity at human bradykinin B1 receptor assessed as inhibition of Des-arg kallidin-induced increase of cytosolic calcium in CHO cells by FLIPR
- ChEMBL_748609 (CHEMBL1780462) Antagonist activity at human bradykinin B1 receptor expressed in CHO cells assessed as inhibition of agonist-induced calcium efflux by aquerin based assay
- ChEMBL_1550092 (CHEMBL3756038) Inhibition of Bacillus thermoproteolyticus thermolysin pretreated with compound for 1 hr followed by the addition of 3.33 uM bradykinin like substrate by spectrofluorometry assay
- ChEMBL_303777 (CHEMBL830134) Binding affinity of the [35S]- radiolabeled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_303778 (CHEMBL830135) Binding affinity of the [35S]- radiolabeled compound to human Bradykinin receptor B2 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_1856689 (CHEMBL4357418) Binding affinity to NT-495 fluorophore reagent labelled human BK beta4 (45 to 166 residues) incubated for 30 mins by microscale thermophoresis assay
- ChEMBL_40108 (CHEMBL657643) Compound was evaluated for inhibitory activity against [3H]BK in membrane preparations from a stable cell line that expresses the human B2 receptor
- ChEMBL_830849 (CHEMBL2065355) Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence analysis
- ChEMBL_830850 (CHEMBL2065356) Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence analysis
- ChEMBL_830851 (CHEMBL2065357) Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence analysis
- ChEMBL_429974 (CHEMBL917704) Antagonist activity at bradykinin B1 receptor expressed in CHO cells assessed as inhibition of des-arg10-kallidin-induced increase in cytosolic calcium level by FLIPR assay
- ChEMBL_885183 (CHEMBL2213717) Inhibition of HCV genotype 1b BK NS3/4A protease D168V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay
- ChEMBL_885185 (CHEMBL2213719) Inhibition of HCV genotype 1b BK NS3/4A protease A156V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay
- ChEMBL_885186 (CHEMBL2213720) Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay
- ChEMBL_797692 (CHEMBL1943912) Antagonist activity at human bradykinin B1 receptor expressed in CHO-D-/aequorin cells assessed as inhibition of DAK-induced intracellular calcium level after 1.5 to 2 hrs by luminometry analysis
- ChEMBL_1856724 (CHEMBL4357453) Inhibition of human BK alpha/beta4 channel expressed in HEK293T cells assessed as inhibition of K+ outward current fraction at -80 mV holding potential by whole cell patch clamp technique
- ChEMBL_1687679 (CHEMBL4038158) Inhibition of human plasma kallikrein spiked in pig vitreous using HMWK as substrate assessed as suppression of bradykinin release preincubated for 15 mins followed by substrate addition measured after 15 mins by ELISA
- ChEMBL_1746012 (CHEMBL4180522) Inhibition of wild-type HCV genotype 1b BK NS5B Cdelta55 RNA dependent RNA polymerase using RNA as substrate after 1 hr in presence of NTPs by [33P]-CTP based topcount assay
- ChEMBL_1687677 (CHEMBL4038156) Inhibition of plasma kallikrein in human plasma using HMWK as substrate assessed as suppression of kaolin-activated protein induced bradykinin release preincubated for 15 mins followed by substrate addition measured after 15 min by ELISA
- ChEMBL_863255 (CHEMBL2176198) Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substrate incubated for 15 mins prior to substrate addition measured after 15 mins by fluorimetric analysis
- Binding Assay For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kallidin (Des Arg10, Leu9), [3,4-Prolyl-3,4-3H(N)] (PerkinElmer, 1.85-4.44 TBq/mmol) to 40 μg membrane protein containing approximately 1 fmol Bradykinin-1 receptor and incubated for 15 min at 27° C. To determine non-specific binding 10 μM Lys-(Des-Arg9)-Bradykinin (Bachem) was added. Membranes were harvested through GF/B (glass fiber filter; PerkinElmer) plates, equilibrated with 0.5% polyethylenimine, air dried at 50° C. for 2 hr. Radioactivity was determined by counting in a topcounter (NXT Packard). Specific binding was defined as total binding minus nonspecific binding and typically represents about 90-95% of the total binding. Antagonist activity is expressed as Ki: inhibitor concentration required for 50% inhibition of specific binding corrected for the concentration of the radioligand.
- Inhibition Assay The activated cathepsin A was diluted in assay buffer (25 mM MES, pH 5.5, containing 5 mM DTT) and mixed with the test compound (dissolved in assay buffer containing (v/v) 3% DMSO) or, in the control experiments, with the vehicle in a multiple assay plate. After incubation for 15 min at room temperature, as substrate then bradykinin carrying an N-terminal Bodipy FL (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl) label (JPT Peptide Technologies GmbH; dissolved in assay buffer) was added to the mixture. The final concentration of cathepsin A was 833 ng/ml and the final concentration of labeled bradykinin 2 μM. After incubation for 15 min at room temperature the reaction was stopped by the addition of stop buffer (130 mM 2-(4-(2-hydroxy-ethyl)-piperazin-1-yl)-ethanesulfonic acid, pH 7.4, containing (v/v) 0.013% Triton X-100, 0.13% Coating Reagent 3 (Caliper Life Sciences), 6.5% DMSO and 20 μM ebelactone B (Sigma, #E0886)).
- Inhibition Assay The activated cathepsin A was diluted in assay buffer (25 mM MES, pH 5.5, containing 5 mM DTT) and mixed with the test compound (dissolved in assay buffer containing (v/v) 3% DMSO) or, in the control experiments, with the vehicle in a multiple assay plate. After incubation for 15 min at room temperature, as substrate then bradykinin carrying an N-terminal Bodipy FL (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl) label (JPT Peptide Technologies GmbH; dissolved in assay buffer) was added to the mixture. The final concentration of cathepsin A was 833 ng/ml and the final concentration of labeled bradykinin 2 μM. After incubation for 15 min at room temperature the reaction was stopped by the addition of stop buffer (130 mM 2-(4-(2-hydroxy-ethyl)-piperazin-1-yl)-ethanesulfonic acid, pH 7.4, containing (v/v) 0.013%° Triton X-100, 0.13% Coating Reagent 3 (Caliper Life Sciences), 6.5% DMSO and 20 μM ebelactone B (Sigma, #E0886)).
- Calcium Flux Assays (hB1 IC50 IMR90) Notably, Compound Examples are tested in the FLIPR assays in the presence (hB1 free IC50) of 0.1% BSA in assay buffer, in order to assess the potency shifts due to serum protein binding of Compound Examples. The effect of BSA on the potency of endothelin receptor antagonists have been described in the prior art (Wu-Wong, J. R. et al. (1997), JPET 281: 791-798). The teaching can be applied in analogy to testing the potency of Bradykinin B1 receptor antagonist in the FLIPR assays. For the calcium flux assay, 80% confluent cells are detached from the culture vessels with Versene (Gibco), and seeded into 384-well plates (Cell binding Surface; Corning, N.Y.; #3683) at a density of 15,000 cells per well. Cells are seeded in a volume of 50 μL in medium without antibiotics and incubated overnight in a humidified atmosphere with 5% CO2 at 37° C. The following day, the medium is replaced with 20 μL of 5 μM Fluo-4AM dye (Molecular Probes) in assay buffer (2.5 mM probenicid, 1 mg/mL pluronic acid, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl, 1 mM MgCl2, 10 mM HEPES, 5.6 mM glucose, and 0.05% gelatine, pH 7.4), which contains or lacks 0.1% BSA for determination of compound potency units as hB1 IC50 or hB1 free IC50, respectively. The calcium indicator loaded cells are incubated at 37° C. for 2 hrs. Extracellular dye is then removed and each well is filled with 45 μL of assay buffer. Cell plates are kept in dark until used. Compound examples are assayed at 8 concentrations in triplicate. Serial 10-fold dilutions in 100% DMSO are made at a 100-times higher concentration than the final concentration, and then diluted 1:10 in assay buffer. 5 μL of each diluted compound is added to the well of cell plates (yielding final concentration with 1% DMSO), and incubated for 30 min at 28° C. before the addition of Bradykinin B1 receptor agonist on the FLIPR instrument. Agonist plates contain the agonist Lys-(Des-Arg)-Bradykinin (Bachem, Brackley) at 3.5×EC90 in assay buffer with 1% DMSO. The addition of agonist 20 μl per well to the assay plate is carried out on the FLIPR instrument while continuously monitoring Ca2+-dependent fluorescence at 538 nm. A peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin (Bachem, Brackley) at 20 □M is used to determine the full inhibition as control. Peak fluorescence is used to determine the response to agonist obtained at each concentration of Compound Examples by the following equation: % Response=100*(RFU(compound)−RFU(control))/(RFU(DMSO)−RFU(control)) RFU means relative fluorescence units. Control means full inhibition by the peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin at 20 □M.The response values are plotted against the logarithm of the compound concentrations. The Compound Examples are tested in triplicates per plate and mean values are plotted in Excel XLfit to determine IC50 values, percentage of maximal inhibition and the Hill slopes.
- Calcium Flux Assays (hB1 IC50 free) Notably, Compound Examples are tested in the FLIPR assays in the absence (hB1 free IC50) of 0.1% BSA in assay buffer, in order to assess the potency shifts due to serum protein binding of Compound Examples. The effect of BSA on the potency of endothelin receptor antagonists have been described in the prior art (Wu-Wong, J. R. et al. (1997), JPET 281: 791-798). The teaching can be applied in analogy to testing the potency of Bradykinin B1 receptor antagonist in the FLIPR assays. For the calcium flux assay, 80% confluent cells are detached from the culture vessels with Versene (Gibco), and seeded into 384-well plates (Cell binding Surface; Corning, N.Y.; #3683) at a density of 15,000 cells per well. Cells are seeded in a volume of 50 μL in medium without antibiotics and incubated overnight in a humidified atmosphere with 5% CO2 at 37° C. The following day, the medium is replaced with 20 μL of 5 μM Fluo-4AM dye (Molecular Probes) in assay buffer (2.5 mM probenicid, 1 mg/mL pluronic acid, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl, 1 mM MgCl2, 10 mM HEPES, 5.6 mM glucose, and 0.05% gelatine, pH 7.4), which contains or lacks 0.1% BSA for determination of compound potency units as hB1 IC50 or hB1 free IC50, respectively. The calcium indicator loaded cells are incubated at 37° C. for 2 hrs. Extracellular dye is then removed and each well is filled with 45 μL of assay buffer. Cell plates are kept in dark until used. Compound examples are assayed at 8 concentrations in triplicate. Serial 10-fold dilutions in 100% DMSO are made at a 100-times higher concentration than the final concentration, and then diluted 1:10 in assay buffer. 5 μL of each diluted compound is added to the well of cell plates (yielding final concentration with 1% DMSO), and incubated for 30 min at 28° C. before the addition of Bradykinin B1 receptor agonist on the FLIPR instrument. Agonist plates contain the agonist Lys-(Des-Arg)-Bradykinin (Bachem, Brackley) at 3.5×EC90 in assay buffer with 1% DMSO. The addition of agonist 20 μl per well to the assay plate is carried out on the FLIPR instrument while continuously monitoring Ca2+-dependent fluorescence at 538 nm. A peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin (Bachem, Brackley) at 20 □M is used to determine the full inhibition as control. Peak fluorescence is used to determine the response to agonist obtained at each concentration of Compound Examples by the following equation: % Response=100*(RFU(compound)−RFU(control))/(RFU(DMSO)−RFU(control)) RFU means relative fluorescence units. Control means full inhibition by the peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin at 20 □M.The response values are plotted against the logarithm of the compound concentrations. The Compound Examples are tested in triplicates per plate and mean values are plotted in Excel XLfit to determine IC50 values, percentage of maximal inhibition and the Hill slopes.
- Dose Response Assay Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-18 (control antagonist for CHOK1 TRPA1 cells); column 1I-P: ATP (control for CHOK1 teton cells); column 2 I-P: 2APB (control antagonist for CHOK1/TRPM8 cells). Growth media was removed from the cell plates (20 ul) and 20 ul of the Replacement Buffer was added followed by addition of 25 ul of diluted dye. All three steps were performed using a Plate Washer BioTek 407. The plates were then incubated for 30' at RT. After incubation, both the cell and compound plates were brought to the FLIPR and 20 ul of the diluted compounds/antagonist/bk were transferred to the cell plates by the FLIPR. Plates were then incubated for 30' at room temperature. After 30' incubation, plates were returned to the FLIPR and 20 ul of 4.5× Cinnamaldehyde was added to the cell plates. During the compound addition as well as agonist addition, fluorescence readings were taken simultaneously from all 384 wells of the cell plate every 1.5 seconds. Five readings were taken to establish a stable baseline, then 20 ul of sample was rapidly (30 ul/sec) and simultaneously added to each well of the cell plate. The fluorescence was continuously monitored before, during and after sample/agonist addition for a total elapsed time of 100 seconds (compound addition) and 120 seconds (agonist addition).
- Polymerase Inhibition Assay The ability of the nucleoside triphosphate derivatives to inhibit the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase was measured in a radiolabeled nucleotide incorporation assay modified from the method described in International Publication No. WO2002/057287. Briefly, 50 μL reactions containing 20 mM HEPES (pH 7.3), 7.5 mM DTT, 20 units/mL RNasIN, 1 μM each of ATP, GTP, UTP and CTP, 20 μCi/mL [33P]-CTP, 10 mM MgCl2, 60 mM NaCl, 100 μg/ml BSA, 0.021 μM DCoH heteropolymer RNA template and 5 nM NS5B (1b-BKΔ55) enzyme were incubated at room temperature for 1 hour. Assay was terminated by the addition of 500 mM EDTA (50 μL). The reaction mixture was transferred to a Millipore DE81 filter plate and the incorporation of labeled CTP was determined using Packard TopCount.
- Identification of SV40 T antigen inhibitors: A route to novel anti-viral reagents A biochemical assay using the ADP-Hunter methodology, purified TAg, and ATP to identify compounds that inhibit the ATPase activity of Tag Southern Research's Specialized Biocontainment Screening Center (SRSBSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Probe Centers Network (MLPCN) Assay Provider: Dr. Jeffery Brodsky, University of Pittsburgh Grant number: 1R03MH084077-01 Assay Rationale and Summary: The oncogenic virus Simian Virus 40 (SV40) is a well-characterized model system to examine the underlying mechanisms of growth control and cancer. SV40 is also closely related to two viruses, JC and BK virus, which infect humans, and result in morbidity and mortality in immuno-compromised patients. Although it is controversial whether SV40 also causes disease in humans, the virus has been found in specific tumors but not in the surrounding tissue. Unlike BKV and JCV, SV40 grows relatively well in mammalian cell culture systems; moreover, ~10% of the p
- Identification of SV40 T antigen inhibitors: Cytotoxicity screen of selected hits Southern Research's Specialized Biocontainment Screening Center (SRSBSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Probe Production Centers Network (MLPCN) Assay Provider: Dr. Jeffery Brodsky, University of Pittsburgh Award: R03MH084077-01 Cytoxicity Assay Rationale and Summary: The oncogenic virus Simian Virus 40 (SV40) is a well-characterized model system to examine the underlying mechanisms of growth control and cancer. SV40 is also closely related to two viruses, JC and BK virus, which infect humans, and result in morbidity and mortality in immuno-compromised patients. Although it is controversial whether SV40 also causes disease in humans, the virus has been found in specific tumors but not in the surrounding tissue. Unlike BKV and JCV, SV40 grows relatively well in mammalian cell culture systems; moreover, ~10% of the population examined in one study has antibodies against SV40, probably because the first polio vaccines were contaminated with
- Inhibition Assay To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present invention, a radiolabeled nucleotide incorporation assay was used. This assay is a modified version of the assay described in International Publication No. WO2002/057287. Briefly, 50 μL reactions containing 20 mM HEPES (pH 7.3); 7.5 mM DTT; 20 units/ml RNasIN; 1 μM each of ATP, GTP, UTP and CTP; 20 μCi/mL [33P]-CTP; 10 mM MgCl; 60 mM NaCl; 100 μg/ml BSA; 0.021 μM DCoH heteropolymer RNA template; and 5 nM NS5B (1b-BKΔ55) enzyme were incubated at room temperature for 1 hour. The assay was then terminated by the addition of 500 mM EDTA (50 μL). The reaction mixture was transferred to a Millipore DE81 filter plate and the incorporation of labeled CTP is determined using Packard TopCount.
- A confirmatory biochemical assay using the ADP-Hunter methodology, purified TAg, and ATP to quantify activity of synthesized compounds that inhibit the ATPase activity of Tag (3) Southern Research's Specialized Biocontainment Screening Center (SRSBSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Probe Centers Network (MLPCN) Assay Provider: Dr. Jeffery Brodsky, University of Pittsburgh Grant number: 1R03MH084077-01 The oncogenic virus Simian Virus 40 (SV40) is a well-characterized model system to examine the underlying mechanisms of growth control and cancer. SV40 is also closely related to two viruses, JC and BK virus, which infect humans, and result in morbidity and mortality in immuno-compromised patients. Although it is controversial whether SV40 also causes disease in humans, the virus has been found in specific tumors but not in the surrounding tissue. Unlike BKV and JCV, SV40 grows relatively well in mammalian cell culture systems; moreover, ~10% of the population examined in one study has antibodies against SV40, probably because the first polio vaccines were contaminated with this virus. The long-term effects of SV40
- Inhibition Assay To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present invention, a radiolabeled nucleotide incorporation assay was used. This assay is a modified version of the assay described in International Publication No. WO2002/057287. Briefly, 50 uL reactions containing 20 mM HEPES (pH 7.3); 7.5 mM DTT; 20 units/ml RNasIN; 1 uM each of ATP, GTP, UTP and CTP; 20 uCi/mL [33P]-CTP; 10 mM MgCl; 60 mM NaCl; 100 ug/ml BSA; 0.021 uM DCoH heteropolymer RNA template; and 5 nM NS5B (1b-BKΔ55) enzyme were incubated at room temperature for 1 hour. The assay was then terminated by the addition of 500 mM EDTA (50 uL). The reaction mixture was transferred to a Millipore DE81 filter plate and the incorporation of labeled CTP is determined using Packard TopCount. Compound IC50 values can then be calculated from experiments with 10 serial 3-fold dilutions of the inhibitor in duplicate. The intrinsic potency (Ki) of an NTP inhibitor is derived from its NS5B IC50 using the Cheng-Prusoff equation for a competitive inhibitor, as described in Cheng et al., Biochem Pharmacol 22:3099-3108 (1973): Ki=IC50/(1+[S]/Km), where [S]=1 uM, and Km is the concentration of cognate NTP yielding half-maximal enzyme activity in the assay absent exogenous inhibitors.
- Dose Response Assay Harvested cells with 0.025% trypsin/EDTA. Resuspended cells in growth media without selection antibiotics. Measured cell density and diluted to 2.4×10^5 cells/ml in media containing 1 μg/ml Doxycycline Plate 25 μl/well into 384 well black/clear tissue culture-treated plates. Incubated overnight at 37° C. Growth media was removed from the cell plates (20 μl) and 20 μl of the Replacement Buffer was added followed by addition of 25 μl of diluted dye. All three steps were performed using a Plate Washer BioTek 407. The plates were then incubated for 30' at RT. After incubation, both the cell and compound plates were brought to the FLIPR and 20 μl of the diluted compounds/antagonist/bk were transferred to the cell plates by the FLIPR. Plates were then incubated for 30' at room temperature. After 30' incubation, plates were returned to the FLIPR and 20 ul of 4.5× Cinnamaldehyde was added to the cell plates. During the compound addition as well as agonist addition, fluorescence readings were taken simultaneously from all 384 wells of the cell plate every 1.5 seconds. Five readings were taken to establish a stable baseline, then 20 μl of sample was rapidly (30 μl/sec) and simultaneously added to each well of the cell plate. The fluorescence was continuously monitored before, during and after sample/agonist addition for a total elapsed time of 100 seconds (compound addition) and 120 seconds (agonist addition).
 US11767305, Compound (-)-BK-5-MAPB BDBM620429 US20250019357, Compound (-)-BK-5MAPB
US11767305, Compound (-)-BK-5-MAPB BDBM620429 US20250019357, Compound (-)-BK-5MAPB US11767305, Compound (-)-BK-6-MAPB BDBM620431 US20250019357, Compound (-)-BK-6MAPB
US11767305, Compound (-)-BK-6-MAPB BDBM620431 US20250019357, Compound (-)-BK-6MAPB US20250019357, Compound (+)-BK-5MAPB BDBM620430 US11767305, Compound (+)-BK-5-MAPB
US20250019357, Compound (+)-BK-5MAPB BDBM620430 US11767305, Compound (+)-BK-5-MAPB US20250019357, Compound (+)-BK-6MAPB BDBM620432 US11767305, Compound (+)-BK-6-MAPB
US20250019357, Compound (+)-BK-6MAPB BDBM620432 US11767305, Compound (+)-BK-6-MAPB BDBM293152 US10106501, Example BK
BDBM293152 US10106501, Example BK US8846656, 17-BK BDBM134489
US8846656, 17-BK BDBM134489 CAS_58-82-2 NSC_105044 Bradykinin BDBM82076
CAS_58-82-2 NSC_105044 Bradykinin BDBM82076 BDBM712824 US20250019357, Compound (-)-BK-6MBPB
BDBM712824 US20250019357, Compound (-)-BK-6MBPB US20250019357, Compound (+)-BK-6MBPB BDBM712825
US20250019357, Compound (+)-BK-6MBPB BDBM712825 BDBM712809 US20250019357, Compound (+)-BK-6-MBPB
BDBM712809 US20250019357, Compound (+)-BK-6-MBPB US20250019357, Compound (-)-BK-6-MBPB BDBM712807
US20250019357, Compound (-)-BK-6-MBPB BDBM712807 BDBM712800 US20250019357, Compound rac-BK-6-MAPB
BDBM712800 US20250019357, Compound rac-BK-6-MAPB BDBM712806 US20250019357, Compound rac-BK-6-MBPB
BDBM712806 US20250019357, Compound rac-BK-6-MBPB US20250019357, Compound rac-BK-5-MAPB BDBM712797
US20250019357, Compound rac-BK-5-MAPB BDBM712797 US20250019357, Compound rac-BK-5-MBPB BDBM712803
US20250019357, Compound rac-BK-5-MBPB BDBM712803 BDBM620426 US11767305, Compound R-5-MBPB US20250019357, Compound (-)-BK-5MBPB
BDBM620426 US11767305, Compound R-5-MBPB US20250019357, Compound (-)-BK-5MBPB US20250019357, Compound (+)-BK-5MBPB BDBM620425 US11767305, Compound S-5-MBPB
US20250019357, Compound (+)-BK-5MBPB BDBM620425 US11767305, Compound S-5-MBPB Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35
Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35 (bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)
(bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)