 BDBM25757 CGP 12177
BDBM25757 CGP 12177 CGP-35949 BDBM50228307
CGP-35949 BDBM50228307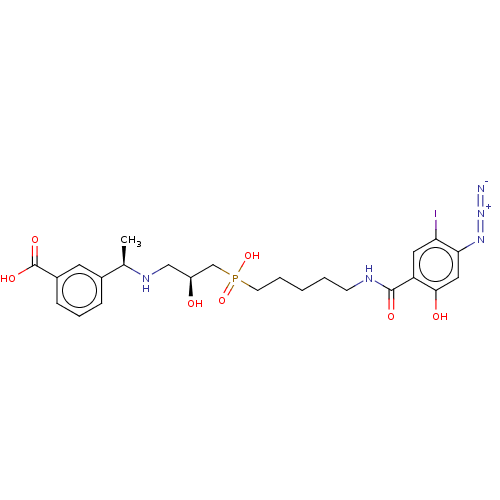 BDBM50108997 CHEMBL1628548 CGP-71872
BDBM50108997 CHEMBL1628548 CGP-71872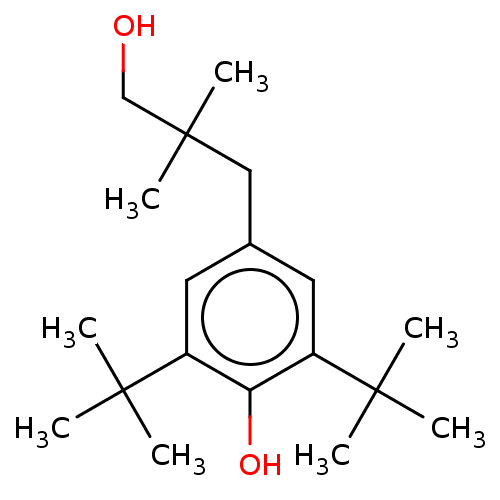 CGP-7930 BDBM50108996 CHEMBL1256697
CGP-7930 BDBM50108996 CHEMBL1256697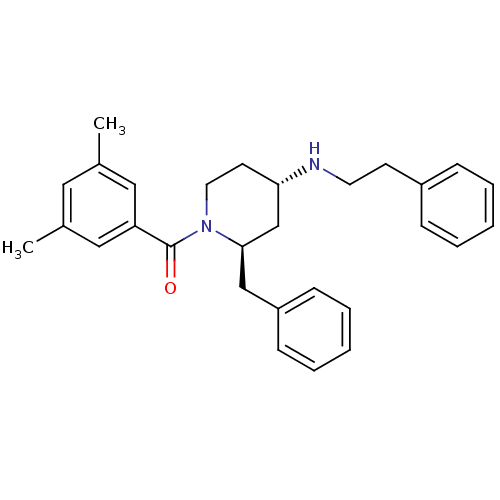 CHEMBL121852 CGP-47899 BDBM50408471
CHEMBL121852 CGP-47899 BDBM50408471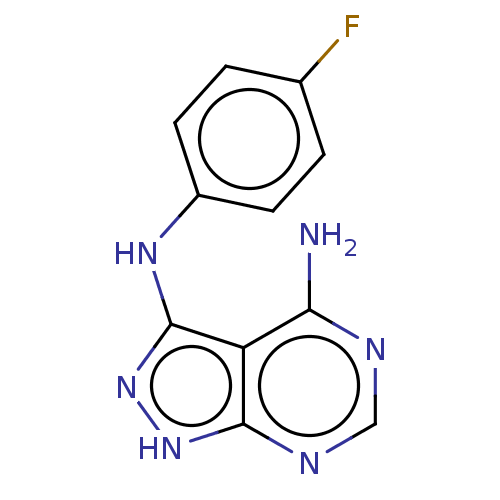 CHEMBL1240885 CGP-57380 BDBM50130693
CHEMBL1240885 CGP-57380 BDBM50130693 CHEMBL411901 BDBM50375662 CGP-74514A
CHEMBL411901 BDBM50375662 CGP-74514A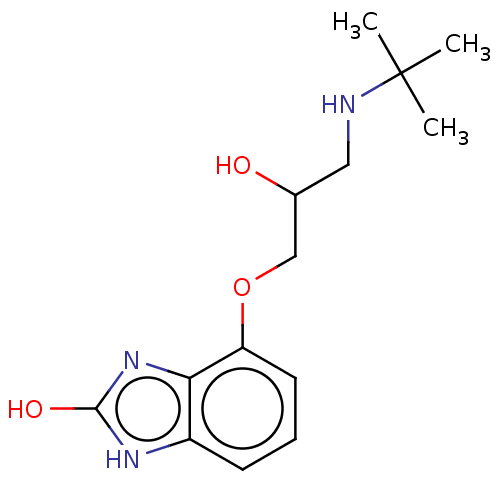 BDBM50027879 CHEBI:73288 CGP-12177
BDBM50027879 CHEBI:73288 CGP-12177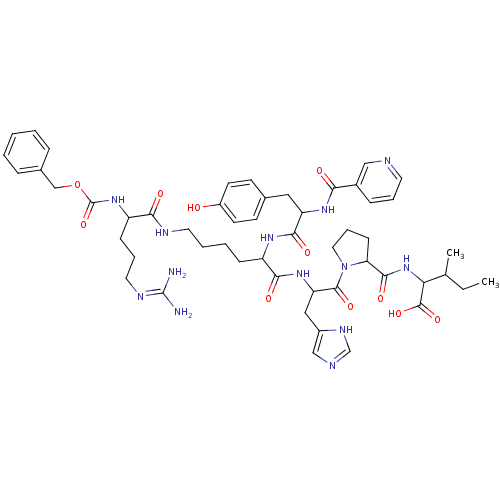 NSC_123794 CAS_123794 BDBM86060 CGP 42112
NSC_123794 CAS_123794 BDBM86060 CGP 42112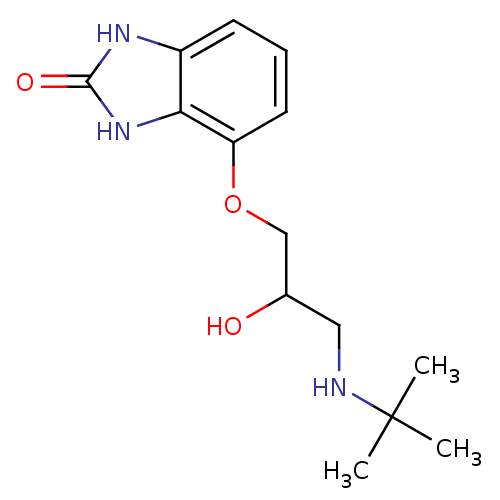 4-[3-(tert-butylamino)-2-hydroxypropoxy]-2,3-dihydro-1H-1,3-benzodiazol-2-one hydrochloride CGP12177 [3H]CGP 12177 BDBM25747 CGP 12177 CGP-12177
4-[3-(tert-butylamino)-2-hydroxypropoxy]-2,3-dihydro-1H-1,3-benzodiazol-2-one hydrochloride CGP12177 [3H]CGP 12177 BDBM25747 CGP 12177 CGP-12177 PKC-412 BDBM50423656 MIDOSTAURIN CGP-41251
PKC-412 BDBM50423656 MIDOSTAURIN CGP-41251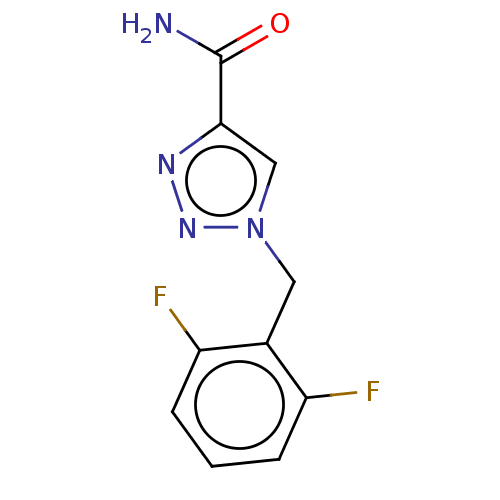 RUF 331 E-2080 Rufinamide Banzel E2080 Inovelon 60231/4 BDBM50515492 RUF-331 CGP 33101 CGP-33101
RUF 331 E-2080 Rufinamide Banzel E2080 Inovelon 60231/4 BDBM50515492 RUF-331 CGP 33101 CGP-33101 NSC_2687 BDBM86452 CGP 12177 CAS_81047-99-6
NSC_2687 BDBM86452 CGP 12177 CAS_81047-99-6 BDBM84736 CGP 20712-A NSC_2685 CAS_81015-67-0
BDBM84736 CGP 20712-A NSC_2685 CAS_81015-67-0 BDBM50419318 YM-08316 BD-40A CGP-25827A FORMOTEROL FUMARATE
BDBM50419318 YM-08316 BD-40A CGP-25827A FORMOTEROL FUMARATE BDBM50033003 CGP-35348 (3-Amino-propyl)-diethoxymethyl-phosphinic acid CHEMBL40157
BDBM50033003 CGP-35348 (3-Amino-propyl)-diethoxymethyl-phosphinic acid CHEMBL40157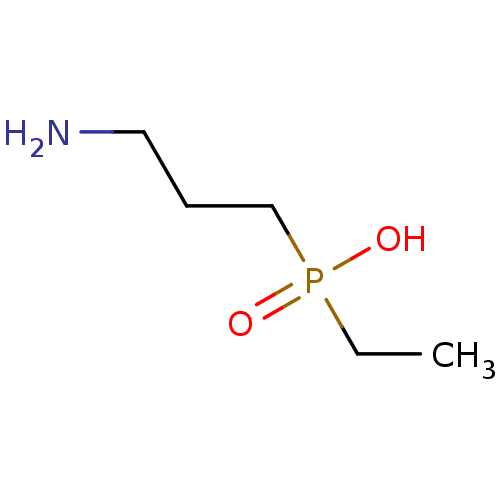 CHEMBL325921 CGP-36216 (3-Amino-propyl)-ethyl-phosphinic acid BDBM50032972
CHEMBL325921 CGP-36216 (3-Amino-propyl)-ethyl-phosphinic acid BDBM50032972 (3-Amino-2-hydroxy-propyl)-methyl-phosphinic acid BDBM50032976 CHEMBL113304 CGP-34938
(3-Amino-2-hydroxy-propyl)-methyl-phosphinic acid BDBM50032976 CHEMBL113304 CGP-34938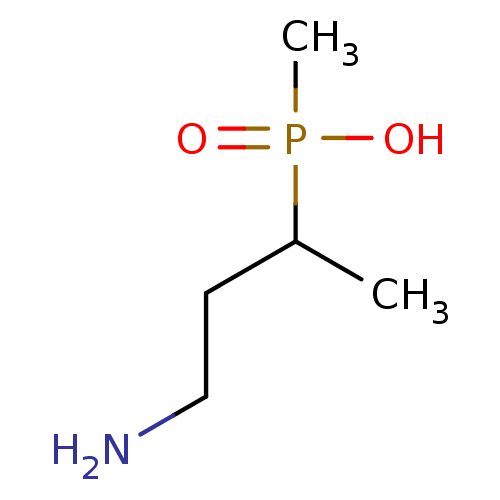 BDBM50032971 CHEMBL113396 CGP-35582 (3-Amino-1-methyl-propyl)-methyl-phosphinic acid
BDBM50032971 CHEMBL113396 CGP-35582 (3-Amino-1-methyl-propyl)-methyl-phosphinic acid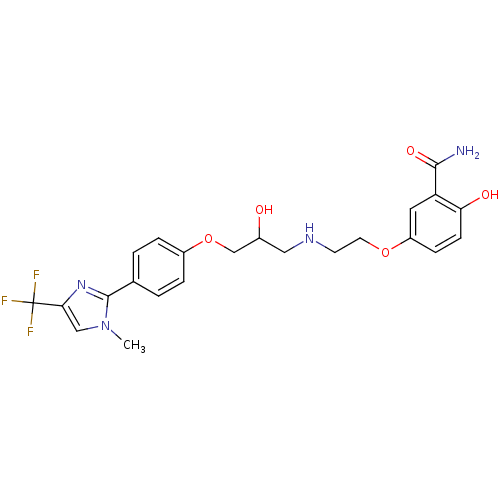 CGP-20712A CGP 20712-A BDBM25746 2-hydroxy-5-{2-[(2-hydroxy-3-{4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy}propyl)amino]ethoxy}benzamide; methanesulfonic acid cid_2685
CGP-20712A CGP 20712-A BDBM25746 2-hydroxy-5-{2-[(2-hydroxy-3-{4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy}propyl)amino]ethoxy}benzamide; methanesulfonic acid cid_2685 4,5-dianilinophthalimide CHEMBL268868 5,6-bis(phenylamino)-1H-isoindole-1,3(2H)-dione BDBM50040929 Cgp 52411
4,5-dianilinophthalimide CHEMBL268868 5,6-bis(phenylamino)-1H-isoindole-1,3(2H)-dione BDBM50040929 Cgp 52411 CHEMBL1240885 N-(4-fluorophenyl)-3H-pyrazolo[3,4-d]pyrimidine-3,4-diamine BDBM50298223 CGP-57380 CHEMBL576817
CHEMBL1240885 N-(4-fluorophenyl)-3H-pyrazolo[3,4-d]pyrimidine-3,4-diamine BDBM50298223 CGP-57380 CHEMBL576817 2-Deamino-L-Abu(1)-L-Lys-L-Asp(NH2)-L-Phe-L-Phe-L-Trp-L-Lys-L-Thr-L-Tyr-L-Thr-L-Ser-L-Abu(1)-OH CGP 23996 CGP-23996 BDBM82467
2-Deamino-L-Abu(1)-L-Lys-L-Asp(NH2)-L-Phe-L-Phe-L-Trp-L-Lys-L-Thr-L-Tyr-L-Thr-L-Ser-L-Abu(1)-OH CGP 23996 CGP-23996 BDBM82467 4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydro-benzoimidazol-2-one BDBM50098668 CHEMBL36060 CGP-12177A
4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydro-benzoimidazol-2-one BDBM50098668 CHEMBL36060 CGP-12177A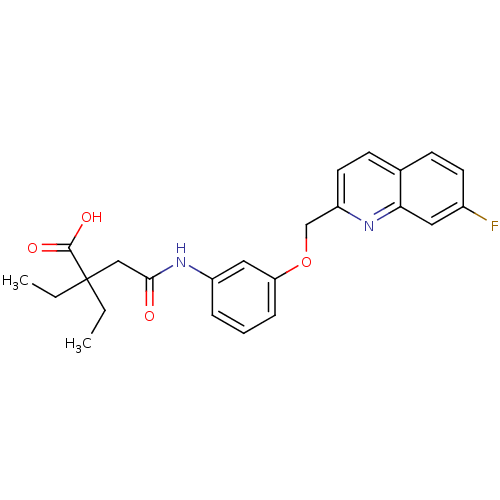 CHEMBL178221 2,2-Diethyl-N-[3-(7-fluoro-quinolin-2-ylmethoxy)-phenyl]-succinamic acid BDBM50068974 CGP-57698
CHEMBL178221 2,2-Diethyl-N-[3-(7-fluoro-quinolin-2-ylmethoxy)-phenyl]-succinamic acid BDBM50068974 CGP-57698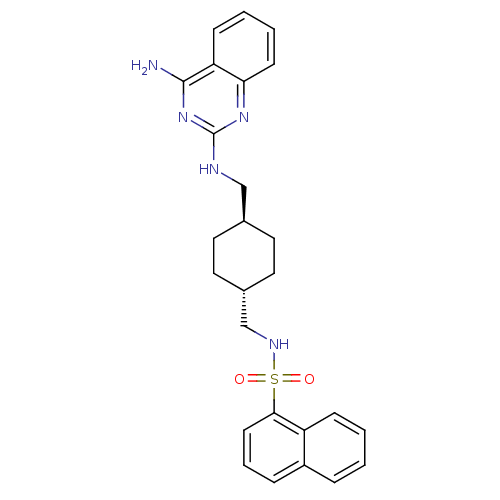 N-{[(1r,4r)-4-{[(4-aminoquinazolin-2-yl)amino]methyl}cyclohexyl]methyl}naphthalene-1-sulfonamide (Compound 1) CGP 71683 CGP-71683A CHEMBL17645 BDBM50089038 Naphthalene-1-sulfonic acid {4-[(4-amino-quinazolin-2-ylamino)-methyl]-cyclohexylmethyl}-amide
N-{[(1r,4r)-4-{[(4-aminoquinazolin-2-yl)amino]methyl}cyclohexyl]methyl}naphthalene-1-sulfonamide (Compound 1) CGP 71683 CGP-71683A CHEMBL17645 BDBM50089038 Naphthalene-1-sulfonic acid {4-[(4-amino-quinazolin-2-ylamino)-methyl]-cyclohexylmethyl}-amide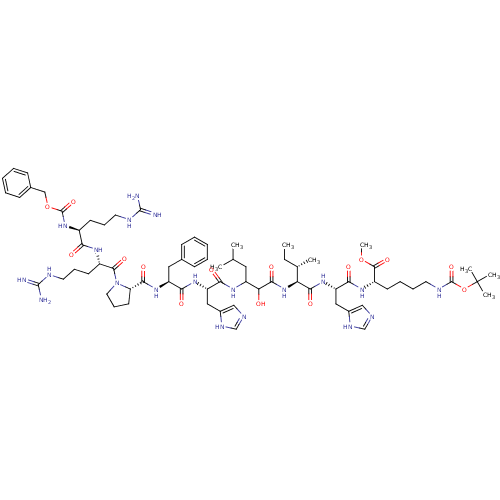 CGP-2928 Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys(N-Boc)-OMe(CGP2928) BDBM50022836 CHEMBL216847
CGP-2928 Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys(N-Boc)-OMe(CGP2928) BDBM50022836 CHEMBL216847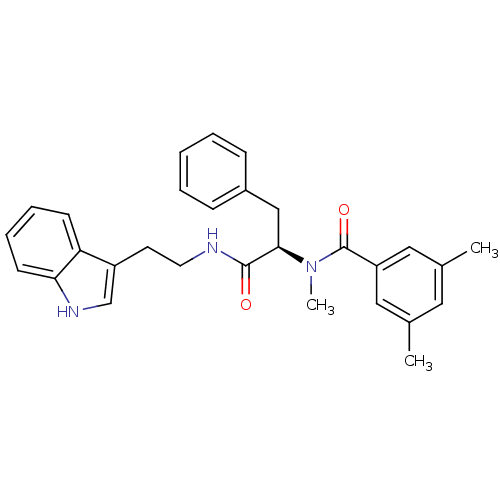 CGP-49941 CHEMBL305615 N-{(R)-1-[2-(1H-Indol-3-yl)-ethylcarbamoyl]-2-phenyl-ethyl}-3,5,N-trimethyl-benzamide BDBM50287878
CGP-49941 CHEMBL305615 N-{(R)-1-[2-(1H-Indol-3-yl)-ethylcarbamoyl]-2-phenyl-ethyl}-3,5,N-trimethyl-benzamide BDBM50287878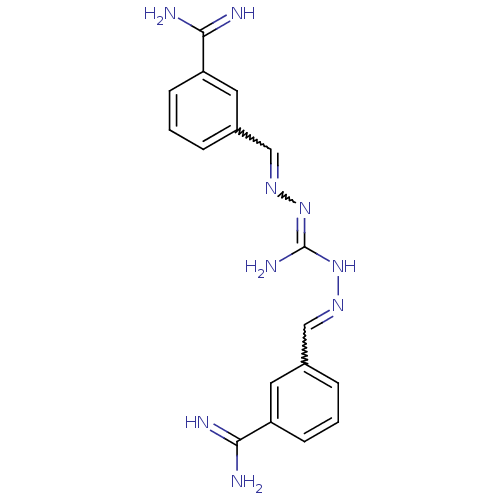 CGP-40215 CHEMBL149797 BDBM50046196 3-((E)-{[(Z)-((2E)-2-{3-[(E)-amino(imino)methyl]benzylidene}hydrazino)(imino)methyl]hydrazono}methyl)benzenecarboximidamide
CGP-40215 CHEMBL149797 BDBM50046196 3-((E)-{[(Z)-((2E)-2-{3-[(E)-amino(imino)methyl]benzylidene}hydrazino)(imino)methyl]hydrazono}methyl)benzenecarboximidamide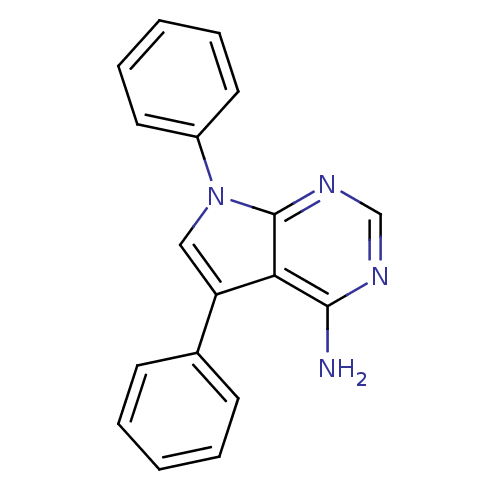 BDBM50088900 CHEMBL169757 CGP-62464 5,7-diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine 5,7-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine
BDBM50088900 CHEMBL169757 CGP-62464 5,7-diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine 5,7-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine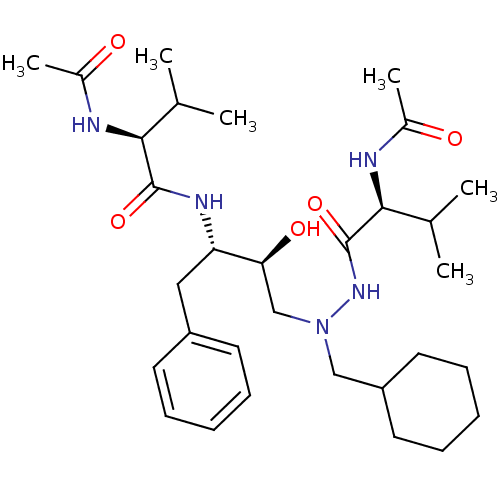 CGP 53820 (2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-acetamido-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]-2-acetamido-3-methylbutanamide BDBM201
CGP 53820 (2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-acetamido-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]-2-acetamido-3-methylbutanamide BDBM201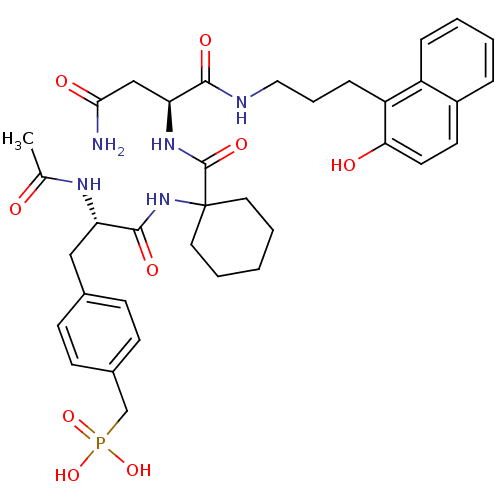 CHEMBL276046 BDBM50099452 CGP-78850 {4-[(S)-2-Acetylamino-2-(1-{(S)-2-carbamoyl-1-[3-(2-hydroxy-naphthalen-1-yl)-propylcarbamoyl]-ethylcarbamoyl}-cyclohexylcarbamoyl)-ethyl]-benzyl}-phosphonic acid
CHEMBL276046 BDBM50099452 CGP-78850 {4-[(S)-2-Acetylamino-2-(1-{(S)-2-carbamoyl-1-[3-(2-hydroxy-naphthalen-1-yl)-propylcarbamoyl]-ethylcarbamoyl}-cyclohexylcarbamoyl)-ethyl]-benzyl}-phosphonic acid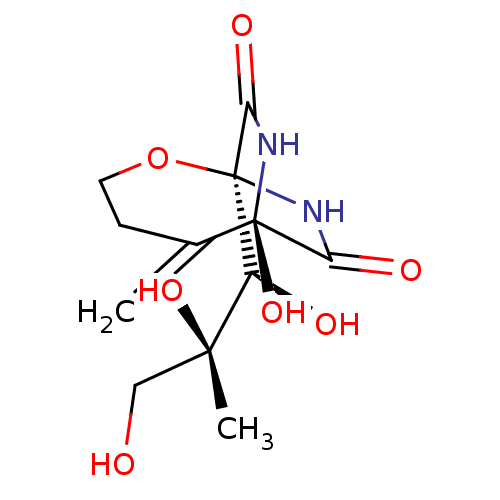 CGP 354E Bicyclomycin BDBM22600 bicozamycin (1S,6R)-6-hydroxy-5-methylidene-1-[(1S,2S)-1,2,3-trihydroxy-2-methylpropyl]-2-oxa-7,9-diazabicyclo[4.2.2]decane-8,10-dione FR 1881
CGP 354E Bicyclomycin BDBM22600 bicozamycin (1S,6R)-6-hydroxy-5-methylidene-1-[(1S,2S)-1,2,3-trihydroxy-2-methylpropyl]-2-oxa-7,9-diazabicyclo[4.2.2]decane-8,10-dione FR 1881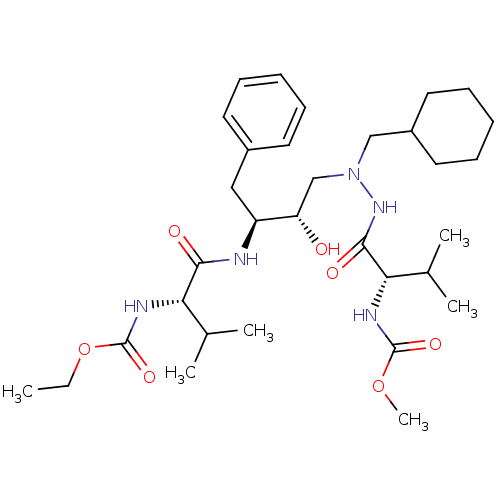 CGP 53820 analog BDBM209 ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
CGP 53820 analog BDBM209 ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CGP 53820 analog BDBM212
2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CGP 53820 analog BDBM212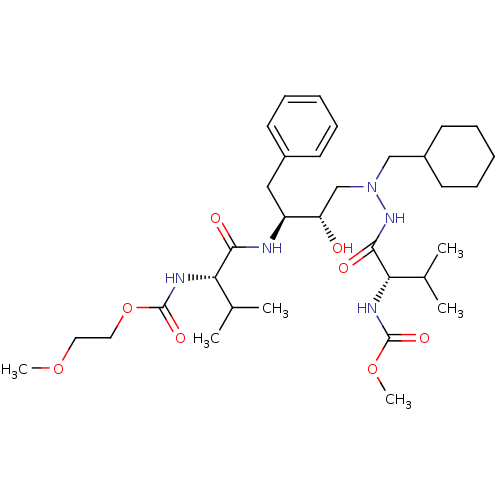 CGP 53820 analog 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM211
CGP 53820 analog 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM211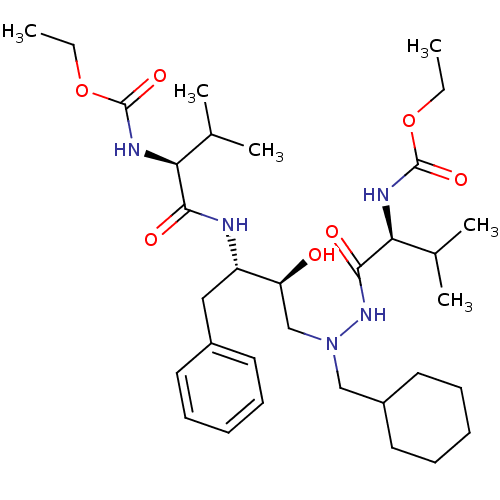 CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CHEMBL324572 BDBM202
CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CHEMBL324572 BDBM202 CGP 53820 analog BDBM208 methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
CGP 53820 analog BDBM208 methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate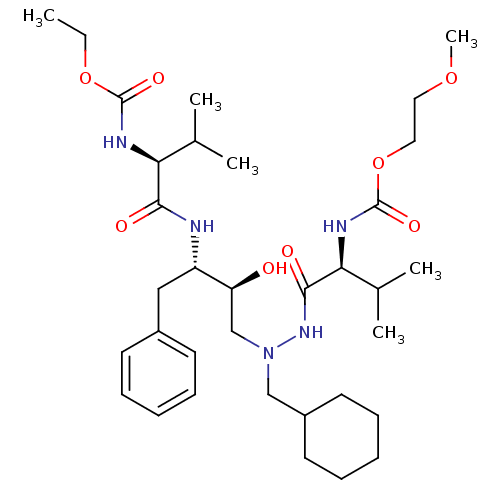 CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM210
CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM210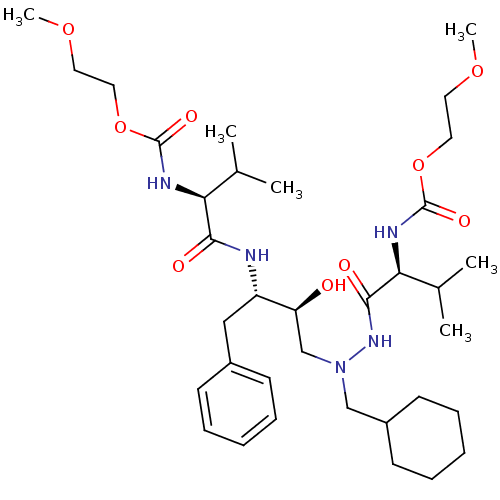 CGP 53820 analog BDBM204 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
CGP 53820 analog BDBM204 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate (2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpropane-2-)sulfonyl]propanamido]-3-(1H-imidazol-4-yl)propanamido]-N-butyl-6-cyclohexyl-4-hydroxy-2-(propan-2-yl)hexanamide BDBM17941 CGP 38560
(2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpropane-2-)sulfonyl]propanamido]-3-(1H-imidazol-4-yl)propanamido]-N-butyl-6-cyclohexyl-4-hydroxy-2-(propan-2-yl)hexanamide BDBM17941 CGP 38560 BDBM50287397 CGP-49823 {(R)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone CHEMBL290364 {(2R,4S)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone
BDBM50287397 CGP-49823 {(R)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone CHEMBL290364 {(2R,4S)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone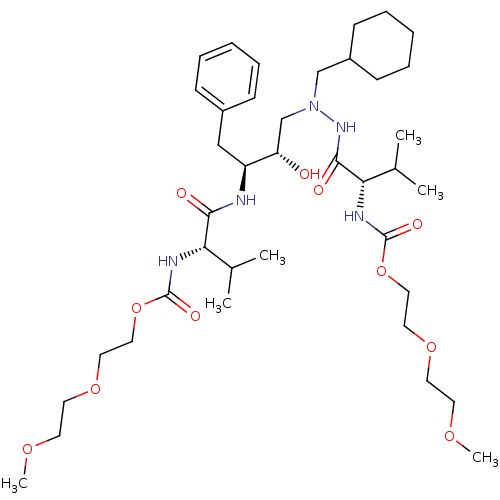 BDBM205 CGP 53820 analog 2-(2-methoxyethoxy)ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-3-methyl-2-(2-oxo-3,6,9-trioxa-1-azadecan-1-yl)butanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
BDBM205 CGP 53820 analog 2-(2-methoxyethoxy)ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-3-methyl-2-(2-oxo-3,6,9-trioxa-1-azadecan-1-yl)butanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate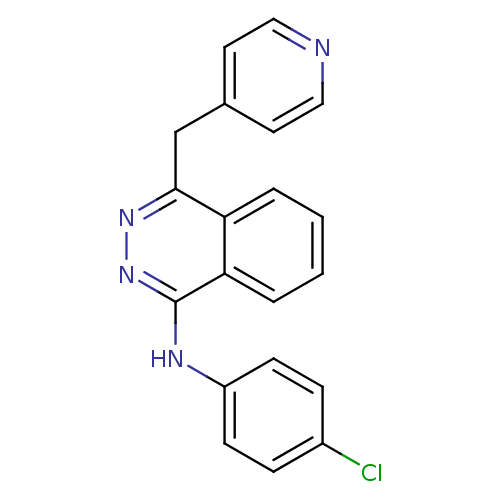 (4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-yl]amine CHEMBL101253 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine CHEMBL75232 BDBM4851 ZK222584 (A) PTK787 PTK787 CGP 79787 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)-1-phthalazinamine cid_151194 PTK-787
(4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-yl]amine CHEMBL101253 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine CHEMBL75232 BDBM4851 ZK222584 (A) PTK787 PTK787 CGP 79787 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)-1-phthalazinamine cid_151194 PTK-787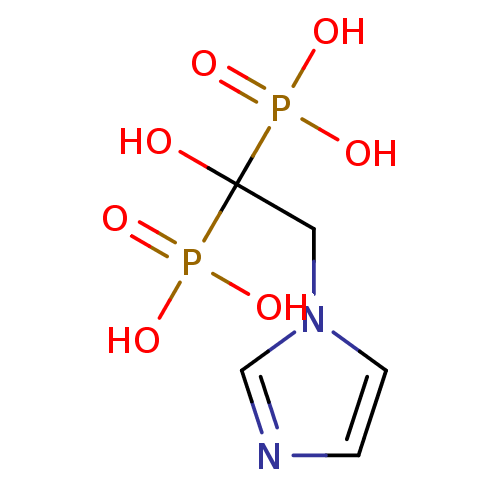 Bisphosphonate 3 Zoledronate CHEMBL924 ZOL zoledronic acid BDBM12578 Reclast Zometa CGP-42446 JMC515594 Compound 55 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid [1-hydroxy-2-(1H-imidazol-1-yl)-1-phosphonoethyl]phosphonic acid US11279719, Example Zolendronic acid (ZOL)
Bisphosphonate 3 Zoledronate CHEMBL924 ZOL zoledronic acid BDBM12578 Reclast Zometa CGP-42446 JMC515594 Compound 55 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid [1-hydroxy-2-(1H-imidazol-1-yl)-1-phosphonoethyl]phosphonic acid US11279719, Example Zolendronic acid (ZOL) CGP-42112A (2S,3R)-2-({(S)-1-[(S)-2-[(S)-2-[(S)-2-Benzoylamino-3-(4-hydroxy-phenyl)-propionylamino]-6-((S)-2-benzyloxycarbonylamino-5-guanidino-pentanoylamino)-hexanoylamino]-3-(3H-imidazol-4-yl)-propionyl]-pyrrolidine-2-carbonyl}-amino)-3-methyl-pentanoic acid CHEMBL267354 BDBM50049202
CGP-42112A (2S,3R)-2-({(S)-1-[(S)-2-[(S)-2-[(S)-2-Benzoylamino-3-(4-hydroxy-phenyl)-propionylamino]-6-((S)-2-benzyloxycarbonylamino-5-guanidino-pentanoylamino)-hexanoylamino]-3-(3H-imidazol-4-yl)-propionyl]-pyrrolidine-2-carbonyl}-amino)-3-methyl-pentanoic acid CHEMBL267354 BDBM50049202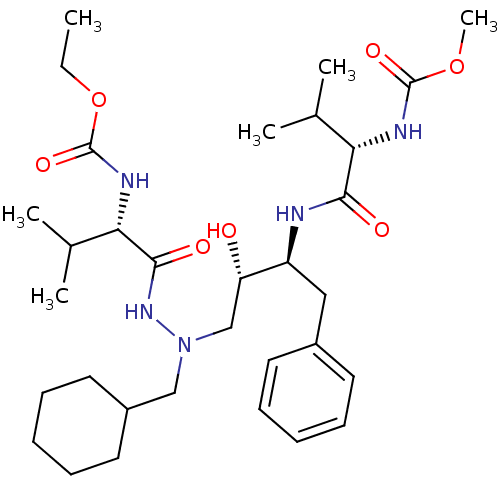 CGP 53820 analog methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM207 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(methoxycarbonyl)-L-valinyl]amino]-6-phenyl-2-azahexane
CGP 53820 analog methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM207 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(methoxycarbonyl)-L-valinyl]amino]-6-phenyl-2-azahexane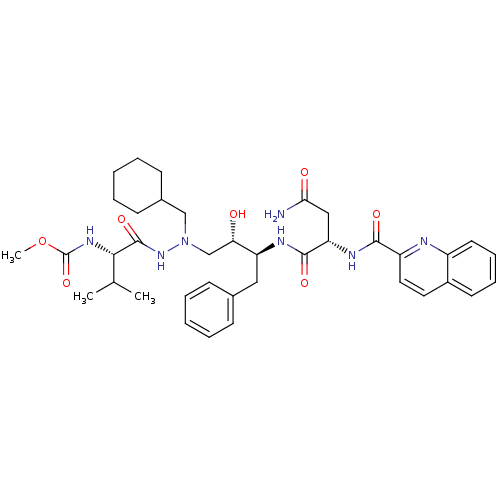 BDBM206 CGP 53820 analog methyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-N-[[(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane
BDBM206 CGP 53820 analog methyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-N-[[(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane CGP 53820 analog BDBM215 1-Cyclohexyl-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane benzyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate
CGP 53820 analog BDBM215 1-Cyclohexyl-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane benzyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate CGP 53820 analog BDBM218 ethyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane
CGP 53820 analog BDBM218 ethyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane BDBM216 2-Methyl-4-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane CGP 53820 analog benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate
BDBM216 2-Methyl-4-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane CGP 53820 analog benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate CGP 53820 analog 2-Methyl-4-[[N-(methoxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane BDBM213
methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate CGP 53820 analog 2-Methyl-4-[[N-(methoxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane BDBM213 (4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid (CGP 79787D) BDBM50121981 (4-chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine succinic acid CHEMBL75232 (4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid(PTK87) N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine succinate
(4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid (CGP 79787D) BDBM50121981 (4-chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine succinic acid CHEMBL75232 (4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid(PTK87) N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine succinate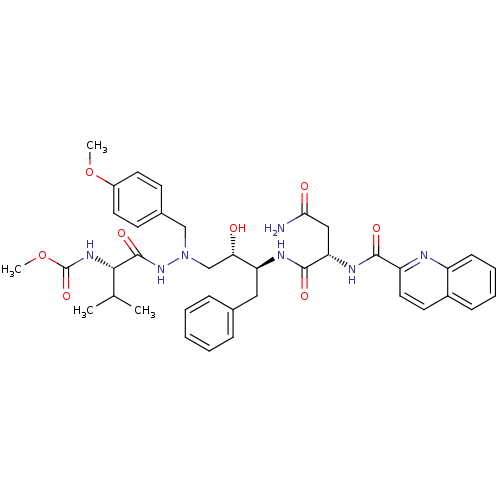 BDBM214 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate
BDBM214 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate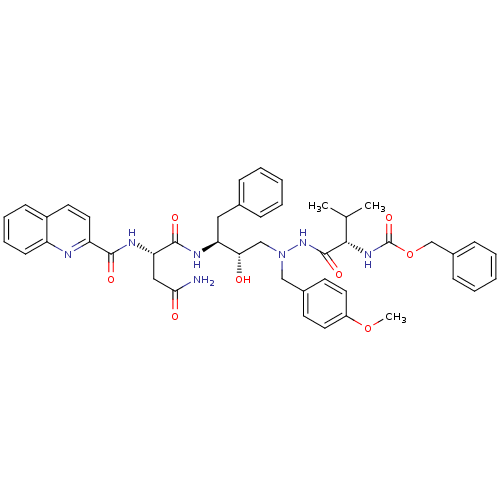 benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate BDBM217 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane
benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate BDBM217 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane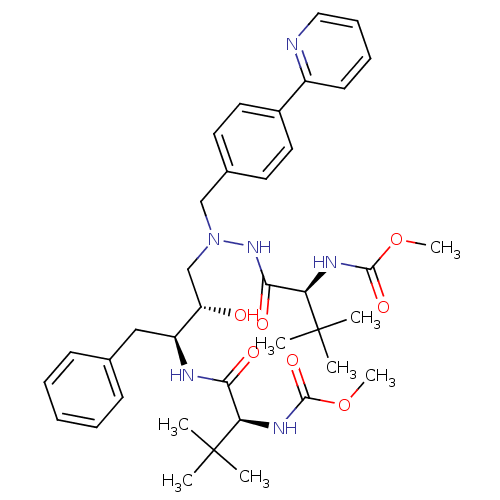 CGP 73547 methyl N-[(1S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate US10806794, Compound Atazanavir US11938127, Compound atazanavir Atazanavir BDBM13934 Latazanavir BMS 232632 CHEMBL1163 methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
CGP 73547 methyl N-[(1S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate US10806794, Compound Atazanavir US11938127, Compound atazanavir Atazanavir BDBM13934 Latazanavir BMS 232632 CHEMBL1163 methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate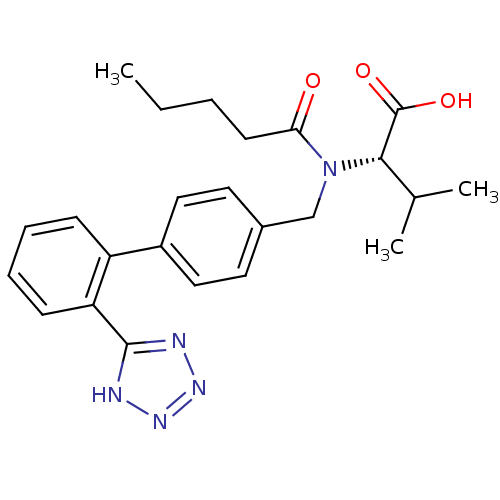 2-{[2'-(2,3-Dihydro-1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-pentanoyl-amino}-3-methyl-butyric acid Exforge (S)-3-Methyl-2-{pentanoyl-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid CGP-48933 CHEMBL1069 BDBM50049186 3-Methyl-2-{((S)-pentanoyl)-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid 3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl]-amino}-butyric acid Diovan Prexxartan (S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid VALSARTAN
2-{[2'-(2,3-Dihydro-1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-pentanoyl-amino}-3-methyl-butyric acid Exforge (S)-3-Methyl-2-{pentanoyl-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid CGP-48933 CHEMBL1069 BDBM50049186 3-Methyl-2-{((S)-pentanoyl)-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid 3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl]-amino}-butyric acid Diovan Prexxartan (S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid VALSARTAN
- Veenstra, SJ; Hauser, K; Schilling, W; Betschart, C; Ofner, S SAR of 2-benzyl-4-aminopiperidines NK1 antagonists. Part 21. synthesis of CGP 49823 Bioorg Med Chem Lett 6: 3029-3034 (1996)
- Ofner, S; Hauser, K; Schilling, W; Vassout, A; Veenstra, SJ SAR of 2-benzyl-4-aminopiperidines: CGP 49823, an orally and centrally active non-peptide NK1 antagonist Bioorg Med Chem Lett 6: 1623-1628 (1996)
- Veenstra, SJ; Hauser, K; Betschart, C Studies on the active conformation of NK1 antagonist CGP 49823. Part 1. Synthesis of conformationally restricted analogs. Bioorg Med Chem Lett 7: 347-350 (1997)
- Veenstra, SJ; Hauser, K; Felber, P Studies on the active conformation of the NK1 antagonist CGP 49823. Part 21. Fluoro-olefin analogs of tertiary amide rotamers. Bioorg Med Chem Lett 7: 351-354 (1997)
- ChEMBL_466538 (CHEMBL928136) Displacement of [3H](-)CGP-12177 from adrenergic beta2 receptor
- ChEMBL_2201478 (CHEMBL5114186) Displacement of [3H]CGP-39653 from NMDA receptor (unknown origin)
- ChEMBL_466537 (CHEMBL928135) Displacement of [3H](-)CGP-12177 from adrenergic beta-1 receptor
- ChEMBL_809479 (CHEMBL2015334) Displacement of [3H](-)-CGP-12177 from adrenergic beta1 receptor by cell based assay
- ChEMBL_643603 (CHEMBL1212467) Displacement of [3H](-)CGP 12177 from human recombinant beta2 receptor expressed in CHO cells
- ChEMBL_68423 (CHEMBL680339) Inhibition of [3H]CGP-27492 binding to cat Gamma-aminobutyric acid type B receptor
- ChEBML_1711421 Displacement of [3H](-)CGP 12177 from human beta1 adrenoceptor after 60 mins by scintillation counting analysis
- ChEBML_35422 The compound was evaluated for the inhibition of binding of [125I]-CGP 42112A to AT2 receptor
- ChEMBL_643602 (CHEMBL1212466) Displacement of [3H](-)CGP 12177 from human recombinant beta-1 receptor expressed in HEK293 cells
- ChEMBL_755353 (CHEMBL1804509) Displacement of [3H]CGP-12177 from human beta2-adrenergic receptor expressed in HEK 293 cells
- ChEMBL_770397 (CHEMBL1833706) Displacement of [3H]-CGP 12177 from human beta-1 adrenergic receptor expressed in CHOK1 cells
- ChEMBL_770398 (CHEMBL1833707) Displacement of [3H]-CGP 12177 from human beta-2 adrenergic receptor expressed in CHOK1 cells
- ChEMBL_770399 (CHEMBL1833708) Displacement of [3H]-CGP 12177 from human beta-3 adrenergic receptor expressed in CHOK1 cells
- ChEMBL_200345 (CHEMBL807247) Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane
- ChEMBL_436826 (CHEMBL905128) Displacement of [3H]CGP-12177 from beta-1 adrenergic receptor in Sprague-Dawley rat cortical membrane
- ChEMBL_68560 (CHEMBL679647) Inhibition of [3H]CGP-27492 binding to Gamma-aminobutyric acid type B receptor of rat cortex
- ChEMBL_68568 (CHEMBL679655) Inhibition of [3H]CGP-27492 binding to Gamma-aminobutyric acid type B receptor of rat cortex
- ChEBML_37547 Tested for binding affinity against beta-1 adrenergic receptor in rat brain using [3H]-CGP- 26505 as radioligand
- ChEMBL_68561 (CHEMBL679648) Inhibition of binding of [3H]CGP-27492 to gamma-aminobutyric acid type B receptor of rat cortex.
- ChEMBL_140702 (CHEMBL751721) Binding affinity of compound was determined against N-methyl-D-aspartate glutamate receptor, using radioligand [3H]- CGP-39653
- ChEMBL_755354 (CHEMBL1804510) Displacement of [3H]CGP-12177 from beta1-adrenergic receptor in Sprague-Dawley rat cortical membrane after 2 hrs
- 3H-CGP 12177 Whole Cell Binding Assay The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined in the presence of 100 nM CGP 20712A for the beta1-adrenoceptor, 100 nM ICI 118551 for the beta2-adrenoceptor, and 100 uM CGP 12177 for the beta3-adrenoceptor. Increasing concentrations of the competing ligand were used until the specific binding of 3H-CGP 12177 was completely inhibited. IC50 was determined as the concentration required to inhibit 50% of the specific binding. From the IC50 value and the known concentration of radioligand 3H-CGP 12177, a Kd (concentration at which half the receptors are bound by the competing ligand) value was calculated using a equation.
- ChEBML_37401 Compound was tested for its binding affinity to human Beta-1 adrenergic receptor by using the radioligand [3H]CGP-12177
- ChEBML_38460 Compound was tested for its binding affinity to human Beta-2 adrenergic receptor by using the radioligand [3H]CGP-12177
- ChEMBL_1283082 (CHEMBL3100638) Displacement of [3H]-CGP-12177 from human beta2 adrenergic receptor expressed in HEK cells by liquid scintillation counting analysis
- ChEMBL_1732886 (CHEMBL4148422) Displacement of [3H]-CGP-12177 from beta1-adrenergic receptor in rat brain cortex after 1 hr by Microbeta scintillation counting method
- ChEMBL_1619402 (CHEMBL3861571) Displacement of [3H]CGP-12177 from beta1 adrenergic receptor in rat brain cerebral cortex after 60 mins by microbeta scintillation counting method
- ChEMBL_1633552 (CHEMBL3876344) Displacement of [125I]CGP 42112A from human recombinant AT2 receptor expressed in HEK293 cells measured after 4 hrs by scintillation counting method
- ChEMBL_1895520 (CHEMBL4397555) Displacement of [3H]-CGP-12177 from murine beta1-adrenergic receptor expressed in HEK293T cells after 60 mins by radioligand competition binding assay
- ChEMBL_1895521 (CHEMBL4397556) Displacement of [3H]-CGP-12177 from human beta2-adrenergic receptor expressed in CHO cells after 60 mins by radioligand competition binding assay
- ChEMBL_1633549 (CHEMBL3876341) Displacement of [3H]CGP 12177 from human recombinant beta1 adrenergic receptor expressed in HEK293 cells measured after 60 mins by scintillation counting method
- ChEMBL_1633550 (CHEMBL3876342) Displacement of [3H]CGP 12177 from human recombinant beta2 adrenergic receptor expressed in CHO cells measured after 120 mins by scintillation counting method
- ChEMBL_1712282 (CHEMBL4122331) Displacement of [3H]-CGP-12177 from adrenergic beta1 receptor in Wistar rat cortex incubated for 60 mins by microbeta liquid scintillation counting analysis
- ChEMBL_770401 (CHEMBL1833710) Antagonist activity at human beta-1 adrenergic receptor site 1 expressed in CGP 12177-stimulated CHO-K1 cells assessed as CRE-SPAP level by fluorescence correlation spectroscopic analysis
- Inhibition Assay Human recombinant adrenergic β2 receptors expressed in CHO cells are used in modified Tris-HCl buffer pH 7.4. A 50 aliquot is incubated with 0.2 nM [3H]CGP-12177 for 60 minutes at 25° C. Non-specific binding is estimated in the presence of 10 μM ICI-118551. Receptors are filtered and washed, the filters are then counted to determine [3H]CGP-12177 specifically bound. Compounds are screened at 10 μM.
- Beta-2 AR binding assay HEK 293 cells stability transfected with cDNA encoding human β2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 0.05% penicillin-streptomycin, and 400 μg/ml G418 as previously described (Pauwels et al., Biochem. Pharmacol. 42: 1683-1689, 1991). The cells were scraped from the 150×25 mm plates and centrifuged at 500×g for 5 minutes. The pellet was homogenized in 50 mM Tris-HCl, pH 7.7, with a Polytron, centrifuged at 27,000×g, and resuspended in the same buffer. The latter process was repeated, and the pellet was resuspended in 25 mM Tris-HCl containing 120 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, and 5 mM glucose, pH 7.4. The binding assays contained 0.3 nM [3H]CGP-12177 in a volume of 1.0 ml. Nonspecific binding was determined by 1 μM propranolol.According to the above-described methods, binding affinities, expressed as Ki values, were determined using membranes obtained from a HEK 293 cell line stably transfected with cDNA encoding human β2-AR (Pauwels et al., Biochem. Pharmacol. 42: 1683-1689, 1991) with [3H]CGP-12177 as the marker ligand. The resulting IC50 values and Hill coefficients were calculated for each test compound using GraphPad Prism software and Ki values were calculated using the Cheng-Prusoff transformation (Biochem Pharmacol 22: 3099-3108, 1973):K i=IC50/(1+L/K d)+ Eqn. 1.Where: L is the concentration of [3H]CGP-12177 and Kd is the binding affinity of the [3H]CGP-12177. Each test compounds was assayed three times.
- AT2 Receptor Binding Assay 15 μL of [125I]CGP 42112A, at a final concentration of 0.05 nM was added to wells of assay plate.Membranes were dispersed using a 21 gauge needle and diluted to the appropriate protein concentration in assay buffer.120 μL membrane suspension (15 μg protein/well) was added to wells of assay plate.Assay plates were incubated at RT for 2 h.Incubations were stopped by rapid filtration through Multiscreen GF/C plates (Millipore, cat. no. MAFCNOB50), using Multiscreen HTS vacuum manifold (Millipore, cat. no. MSVMHTS00) after pre-wetting filters with wash buffer.Filters were washed five times with ice-cold wash buffer.Filters were dried at RT.50 μL MicroScint 40 was added to each well.Bound 125I was determined using MicroBeta scintillation counter in Trilux mode, for 1 min per well.
- Beta2-AR Binding Assay HEK 293 cells stability transfected with cDNA encoding human beta2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 0.05% penicillin-streptomycin, and 400 g/ml G418 as previously described (Pauwels et al., Biochem. Pharmacol. 42: 1683-1689, 1991). The cells were scraped from the 150x25 mm plates and centrifuged at 500xg for 5 minutes. The pellet was homogenized in 50 mM Tris-HCl, pH 7.7, with a Polytron, centrifuged at 27,000xg, and resuspended in the same buffer. The latter process was repeated, and the pellet was resuspended in 25 mM Tris-HCl containing 120 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, and 5 mM glucose, pH 7.4. The binding assays contained 0.3 nM [3H]CGP-12177 in a volume of 1.0 ml. Nonspecific binding was determined by 1 uM propranolol.
- Beta-AR binding assay β1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988). In brief, male Sprague-Dawley rats weighing 250-350 g were decapitated and their brains quickly removed. The cerebral cortices were dissected on ice, weighed and promptly transferred to a 50 ml test tube containing approximately 30 ml of 50 mM Tris-HCl, pH 7.8 (at room temperature). The tissues were homogenized with a polytron and centrifuged at 20,000×g for 12 min at 4° C. The pellet was washed again in the same manner and resuspended at a concentration of 20 mg (original wet wt) per 1 ml in the assay buffer (20 mM Tris-HCl, 10 mM MgCl2, 1 mM EDTA, 0.1 mM ascorbic acid at pH 7.8). To block the β2 sites present in the cortical membrane preparation, 30 nM ICI 118-551 was also added to the assay buffer. To wells containing 100 μl of the test drug and 100 μl of [3H]CGP-12177 (1.4 nM final concentration), 0.8 ml of tissue homogenate was added. After 2 hours at 25° C., the incubation was terminated by rapid filtration. Nonspecific binding was determined by 10 μM propranolol.
- Beta1-AR Binding Assay Beta1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988). In brief, male Sprague-Dawley rats weighing 250-350 g were decapitated and their brains quickly removed. The cerebral cortices were dissected on ice, weighed and promptly transferred to a 50 ml test tube containing approximately 30 ml of 50 mM Tris-HCl, pH 7.8 (at room temperature). The tissues were homogenized with a polytron and centrifuged at 20,000xg for 12 min at 4° C. The pellet was washed again in the same manner and resuspended at a concentration of 20 mg (original wet wt) per 1 ml in the assay buffer (20 mM Tris-HCl, 10 mM MgCl2, 1 mM EDTA, 0.1 mM ascorbic acid at pH 7.8). To block the beta2 sites present in the cortical membrane preparation, 30 nM ICI 118-551 was also added to the assay buffer. To wells containing 100 ul of the test drug and 100 ul of [3H]CGP-12177 (1.4 nM final concentration), 0.8 ml of tissue homogenate was added. After 2 hours at 25° C., the incubation was terminated by rapid filtration. Nonspecific binding was determined by 10 uM propranolol.
 BDBM25757 CGP 12177
BDBM25757 CGP 12177 CGP-35949 BDBM50228307
CGP-35949 BDBM50228307 BDBM50108997 CHEMBL1628548 CGP-71872
BDBM50108997 CHEMBL1628548 CGP-71872 CGP-7930 BDBM50108996 CHEMBL1256697
CGP-7930 BDBM50108996 CHEMBL1256697 CHEMBL121852 CGP-47899 BDBM50408471
CHEMBL121852 CGP-47899 BDBM50408471 CHEMBL1240885 CGP-57380 BDBM50130693
CHEMBL1240885 CGP-57380 BDBM50130693 CHEMBL411901 BDBM50375662 CGP-74514A
CHEMBL411901 BDBM50375662 CGP-74514A BDBM50027879 CHEBI:73288 CGP-12177
BDBM50027879 CHEBI:73288 CGP-12177 NSC_123794 CAS_123794 BDBM86060 CGP 42112
NSC_123794 CAS_123794 BDBM86060 CGP 42112 4-[3-(tert-butylamino)-2-hydroxypropoxy]-2,3-dihydro-1H-1,3-benzodiazol-2-one hydrochloride CGP12177 [3H]CGP 12177 BDBM25747 CGP 12177 CGP-12177
4-[3-(tert-butylamino)-2-hydroxypropoxy]-2,3-dihydro-1H-1,3-benzodiazol-2-one hydrochloride CGP12177 [3H]CGP 12177 BDBM25747 CGP 12177 CGP-12177 PKC-412 BDBM50423656 MIDOSTAURIN CGP-41251
PKC-412 BDBM50423656 MIDOSTAURIN CGP-41251 RUF 331 E-2080 Rufinamide Banzel E2080 Inovelon 60231/4 BDBM50515492 RUF-331 CGP 33101 CGP-33101
RUF 331 E-2080 Rufinamide Banzel E2080 Inovelon 60231/4 BDBM50515492 RUF-331 CGP 33101 CGP-33101 NSC_2687 BDBM86452 CGP 12177 CAS_81047-99-6
NSC_2687 BDBM86452 CGP 12177 CAS_81047-99-6 BDBM84736 CGP 20712-A NSC_2685 CAS_81015-67-0
BDBM84736 CGP 20712-A NSC_2685 CAS_81015-67-0 BDBM50419318 YM-08316 BD-40A CGP-25827A FORMOTEROL FUMARATE
BDBM50419318 YM-08316 BD-40A CGP-25827A FORMOTEROL FUMARATE BDBM50033003 CGP-35348 (3-Amino-propyl)-diethoxymethyl-phosphinic acid CHEMBL40157
BDBM50033003 CGP-35348 (3-Amino-propyl)-diethoxymethyl-phosphinic acid CHEMBL40157 CHEMBL325921 CGP-36216 (3-Amino-propyl)-ethyl-phosphinic acid BDBM50032972
CHEMBL325921 CGP-36216 (3-Amino-propyl)-ethyl-phosphinic acid BDBM50032972 (3-Amino-2-hydroxy-propyl)-methyl-phosphinic acid BDBM50032976 CHEMBL113304 CGP-34938
(3-Amino-2-hydroxy-propyl)-methyl-phosphinic acid BDBM50032976 CHEMBL113304 CGP-34938 BDBM50032971 CHEMBL113396 CGP-35582 (3-Amino-1-methyl-propyl)-methyl-phosphinic acid
BDBM50032971 CHEMBL113396 CGP-35582 (3-Amino-1-methyl-propyl)-methyl-phosphinic acid CGP-20712A CGP 20712-A BDBM25746 2-hydroxy-5-{2-[(2-hydroxy-3-{4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy}propyl)amino]ethoxy}benzamide; methanesulfonic acid cid_2685
CGP-20712A CGP 20712-A BDBM25746 2-hydroxy-5-{2-[(2-hydroxy-3-{4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy}propyl)amino]ethoxy}benzamide; methanesulfonic acid cid_2685 4,5-dianilinophthalimide CHEMBL268868 5,6-bis(phenylamino)-1H-isoindole-1,3(2H)-dione BDBM50040929 Cgp 52411
4,5-dianilinophthalimide CHEMBL268868 5,6-bis(phenylamino)-1H-isoindole-1,3(2H)-dione BDBM50040929 Cgp 52411 CHEMBL1240885 N-(4-fluorophenyl)-3H-pyrazolo[3,4-d]pyrimidine-3,4-diamine BDBM50298223 CGP-57380 CHEMBL576817
CHEMBL1240885 N-(4-fluorophenyl)-3H-pyrazolo[3,4-d]pyrimidine-3,4-diamine BDBM50298223 CGP-57380 CHEMBL576817 2-Deamino-L-Abu(1)-L-Lys-L-Asp(NH2)-L-Phe-L-Phe-L-Trp-L-Lys-L-Thr-L-Tyr-L-Thr-L-Ser-L-Abu(1)-OH CGP 23996 CGP-23996 BDBM82467
2-Deamino-L-Abu(1)-L-Lys-L-Asp(NH2)-L-Phe-L-Phe-L-Trp-L-Lys-L-Thr-L-Tyr-L-Thr-L-Ser-L-Abu(1)-OH CGP 23996 CGP-23996 BDBM82467 4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydro-benzoimidazol-2-one BDBM50098668 CHEMBL36060 CGP-12177A
4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydro-benzoimidazol-2-one BDBM50098668 CHEMBL36060 CGP-12177A CHEMBL178221 2,2-Diethyl-N-[3-(7-fluoro-quinolin-2-ylmethoxy)-phenyl]-succinamic acid BDBM50068974 CGP-57698
CHEMBL178221 2,2-Diethyl-N-[3-(7-fluoro-quinolin-2-ylmethoxy)-phenyl]-succinamic acid BDBM50068974 CGP-57698 N-{[(1r,4r)-4-{[(4-aminoquinazolin-2-yl)amino]methyl}cyclohexyl]methyl}naphthalene-1-sulfonamide (Compound 1) CGP 71683 CGP-71683A CHEMBL17645 BDBM50089038 Naphthalene-1-sulfonic acid {4-[(4-amino-quinazolin-2-ylamino)-methyl]-cyclohexylmethyl}-amide
N-{[(1r,4r)-4-{[(4-aminoquinazolin-2-yl)amino]methyl}cyclohexyl]methyl}naphthalene-1-sulfonamide (Compound 1) CGP 71683 CGP-71683A CHEMBL17645 BDBM50089038 Naphthalene-1-sulfonic acid {4-[(4-amino-quinazolin-2-ylamino)-methyl]-cyclohexylmethyl}-amide CGP-2928 Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys(N-Boc)-OMe(CGP2928) BDBM50022836 CHEMBL216847
CGP-2928 Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys(N-Boc)-OMe(CGP2928) BDBM50022836 CHEMBL216847 CGP-49941 CHEMBL305615 N-{(R)-1-[2-(1H-Indol-3-yl)-ethylcarbamoyl]-2-phenyl-ethyl}-3,5,N-trimethyl-benzamide BDBM50287878
CGP-49941 CHEMBL305615 N-{(R)-1-[2-(1H-Indol-3-yl)-ethylcarbamoyl]-2-phenyl-ethyl}-3,5,N-trimethyl-benzamide BDBM50287878 CGP-40215 CHEMBL149797 BDBM50046196 3-((E)-{[(Z)-((2E)-2-{3-[(E)-amino(imino)methyl]benzylidene}hydrazino)(imino)methyl]hydrazono}methyl)benzenecarboximidamide
CGP-40215 CHEMBL149797 BDBM50046196 3-((E)-{[(Z)-((2E)-2-{3-[(E)-amino(imino)methyl]benzylidene}hydrazino)(imino)methyl]hydrazono}methyl)benzenecarboximidamide BDBM50088900 CHEMBL169757 CGP-62464 5,7-diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine 5,7-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine
BDBM50088900 CHEMBL169757 CGP-62464 5,7-diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine 5,7-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine CGP 53820 (2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-acetamido-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]-2-acetamido-3-methylbutanamide BDBM201
CGP 53820 (2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-acetamido-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]-2-acetamido-3-methylbutanamide BDBM201 CHEMBL276046 BDBM50099452 CGP-78850 {4-[(S)-2-Acetylamino-2-(1-{(S)-2-carbamoyl-1-[3-(2-hydroxy-naphthalen-1-yl)-propylcarbamoyl]-ethylcarbamoyl}-cyclohexylcarbamoyl)-ethyl]-benzyl}-phosphonic acid
CHEMBL276046 BDBM50099452 CGP-78850 {4-[(S)-2-Acetylamino-2-(1-{(S)-2-carbamoyl-1-[3-(2-hydroxy-naphthalen-1-yl)-propylcarbamoyl]-ethylcarbamoyl}-cyclohexylcarbamoyl)-ethyl]-benzyl}-phosphonic acid CGP 354E Bicyclomycin BDBM22600 bicozamycin (1S,6R)-6-hydroxy-5-methylidene-1-[(1S,2S)-1,2,3-trihydroxy-2-methylpropyl]-2-oxa-7,9-diazabicyclo[4.2.2]decane-8,10-dione FR 1881
CGP 354E Bicyclomycin BDBM22600 bicozamycin (1S,6R)-6-hydroxy-5-methylidene-1-[(1S,2S)-1,2,3-trihydroxy-2-methylpropyl]-2-oxa-7,9-diazabicyclo[4.2.2]decane-8,10-dione FR 1881 CGP 53820 analog BDBM209 ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
CGP 53820 analog BDBM209 ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CGP 53820 analog BDBM212
2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CGP 53820 analog BDBM212 CGP 53820 analog 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM211
CGP 53820 analog 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(methoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM211 CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CHEMBL324572 BDBM202
CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CHEMBL324572 BDBM202 CGP 53820 analog BDBM208 methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
CGP 53820 analog BDBM208 methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM210
CGP 53820 analog ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM210 CGP 53820 analog BDBM204 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
CGP 53820 analog BDBM204 2-methoxyethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-{[(2-methoxyethoxy)carbonyl]amino}-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate (2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpropane-2-)sulfonyl]propanamido]-3-(1H-imidazol-4-yl)propanamido]-N-butyl-6-cyclohexyl-4-hydroxy-2-(propan-2-yl)hexanamide BDBM17941 CGP 38560
(2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpropane-2-)sulfonyl]propanamido]-3-(1H-imidazol-4-yl)propanamido]-N-butyl-6-cyclohexyl-4-hydroxy-2-(propan-2-yl)hexanamide BDBM17941 CGP 38560 BDBM50287397 CGP-49823 {(R)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone CHEMBL290364 {(2R,4S)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone
BDBM50287397 CGP-49823 {(R)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone CHEMBL290364 {(2R,4S)-2-Benzyl-4-[(quinolin-4-ylmethyl)-amino]-piperidin-1-yl}-(3,5-dimethyl-phenyl)-methanone BDBM205 CGP 53820 analog 2-(2-methoxyethoxy)ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-3-methyl-2-(2-oxo-3,6,9-trioxa-1-azadecan-1-yl)butanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate
BDBM205 CGP 53820 analog 2-(2-methoxyethoxy)ethyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-3-methyl-2-(2-oxo-3,6,9-trioxa-1-azadecan-1-yl)butanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate (4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-yl]amine CHEMBL101253 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine CHEMBL75232 BDBM4851 ZK222584 (A) PTK787 PTK787 CGP 79787 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)-1-phthalazinamine cid_151194 PTK-787
(4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-yl]amine CHEMBL101253 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine CHEMBL75232 BDBM4851 ZK222584 (A) PTK787 PTK787 CGP 79787 N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)-1-phthalazinamine cid_151194 PTK-787 Bisphosphonate 3 Zoledronate CHEMBL924 ZOL zoledronic acid BDBM12578 Reclast Zometa CGP-42446 JMC515594 Compound 55 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid [1-hydroxy-2-(1H-imidazol-1-yl)-1-phosphonoethyl]phosphonic acid US11279719, Example Zolendronic acid (ZOL)
Bisphosphonate 3 Zoledronate CHEMBL924 ZOL zoledronic acid BDBM12578 Reclast Zometa CGP-42446 JMC515594 Compound 55 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid [1-hydroxy-2-(1H-imidazol-1-yl)-1-phosphonoethyl]phosphonic acid US11279719, Example Zolendronic acid (ZOL) CGP-42112A (2S,3R)-2-({(S)-1-[(S)-2-[(S)-2-[(S)-2-Benzoylamino-3-(4-hydroxy-phenyl)-propionylamino]-6-((S)-2-benzyloxycarbonylamino-5-guanidino-pentanoylamino)-hexanoylamino]-3-(3H-imidazol-4-yl)-propionyl]-pyrrolidine-2-carbonyl}-amino)-3-methyl-pentanoic acid CHEMBL267354 BDBM50049202
CGP-42112A (2S,3R)-2-({(S)-1-[(S)-2-[(S)-2-[(S)-2-Benzoylamino-3-(4-hydroxy-phenyl)-propionylamino]-6-((S)-2-benzyloxycarbonylamino-5-guanidino-pentanoylamino)-hexanoylamino]-3-(3H-imidazol-4-yl)-propionyl]-pyrrolidine-2-carbonyl}-amino)-3-methyl-pentanoic acid CHEMBL267354 BDBM50049202 CGP 53820 analog methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM207 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(methoxycarbonyl)-L-valinyl]amino]-6-phenyl-2-azahexane
CGP 53820 analog methyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-[(ethoxycarbonyl)amino]-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2-methylpropyl]carbamate BDBM207 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(methoxycarbonyl)-L-valinyl]amino]-6-phenyl-2-azahexane BDBM206 CGP 53820 analog methyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-N-[[(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane
BDBM206 CGP 53820 analog methyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-N-[[(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane CGP 53820 analog BDBM215 1-Cyclohexyl-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane benzyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate
CGP 53820 analog BDBM215 1-Cyclohexyl-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane benzyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate CGP 53820 analog BDBM218 ethyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane
CGP 53820 analog BDBM218 ethyl N-[(1S)-1-[N'-(cyclohexylmethyl)-N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]hydrazinecarbonyl]-2-methylpropyl]carbamate 1-Cyclohexyl-2-[[N-(ethoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane BDBM216 2-Methyl-4-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane CGP 53820 analog benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate
BDBM216 2-Methyl-4-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane CGP 53820 analog benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate CGP 53820 analog 2-Methyl-4-[[N-(methoxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane BDBM213
methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-(2-methylpropyl)hydrazinecarbonyl}-2-methylpropyl]carbamate CGP 53820 analog 2-Methyl-4-[[N-(methoxycarbonyl)-L-valinyl]amino]-6(S)-hydroxy-7(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-8-phenyl-4-azaoctane BDBM213 (4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid (CGP 79787D) BDBM50121981 (4-chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine succinic acid CHEMBL75232 (4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid(PTK87) N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine succinate
(4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid (CGP 79787D) BDBM50121981 (4-chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine succinic acid CHEMBL75232 (4-Chloro-phenyl)-(4-pyridin-4-ylmethyl-phthalazin-1-yl)-amine; compound with succinic acid(PTK87) N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine succinate BDBM214 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate
BDBM214 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(methoxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane methyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate BDBM217 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane
benzyl N-[(1S)-1-{N'-[(2S,3S)-2-hydroxy-4-phenyl-3-[(2S)-2-(quinolin-2-ylformamido)butanediamido]butyl]-N'-[(4-methoxyphenyl)methyl]hydrazinecarbonyl}-2-methylpropyl]carbamate BDBM217 CGP 53820 analog 1-(4-Methoxyphenyl)-2-[[N-(benzyloxycarbonyl)-L-valinyl]amino]-4(S)-hydroxy-5(S)-[[N-(quinolin-2-ylcarbonyl)-L-asparaginyl]amino]-6-phenyl-2-azahexane CGP 73547 methyl N-[(1S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate US10806794, Compound Atazanavir US11938127, Compound atazanavir Atazanavir BDBM13934 Latazanavir BMS 232632 CHEMBL1163 methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
CGP 73547 methyl N-[(1S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate US10806794, Compound Atazanavir US11938127, Compound atazanavir Atazanavir BDBM13934 Latazanavir BMS 232632 CHEMBL1163 methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate 2-{[2'-(2,3-Dihydro-1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-pentanoyl-amino}-3-methyl-butyric acid Exforge (S)-3-Methyl-2-{pentanoyl-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid CGP-48933 CHEMBL1069 BDBM50049186 3-Methyl-2-{((S)-pentanoyl)-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid 3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl]-amino}-butyric acid Diovan Prexxartan (S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid VALSARTAN
2-{[2'-(2,3-Dihydro-1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-pentanoyl-amino}-3-methyl-butyric acid Exforge (S)-3-Methyl-2-{pentanoyl-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid CGP-48933 CHEMBL1069 BDBM50049186 3-Methyl-2-{((S)-pentanoyl)-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid 3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl]-amino}-butyric acid Diovan Prexxartan (S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric acid VALSARTAN