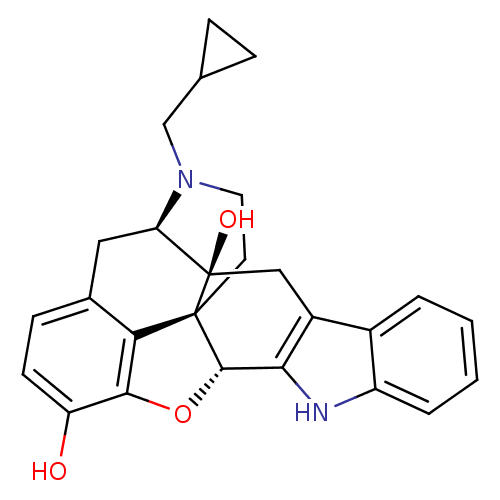
 BDBM50370067 US12215173, Compound Naltrindole CHEMBL1237164
BDBM50370067 US12215173, Compound Naltrindole CHEMBL1237164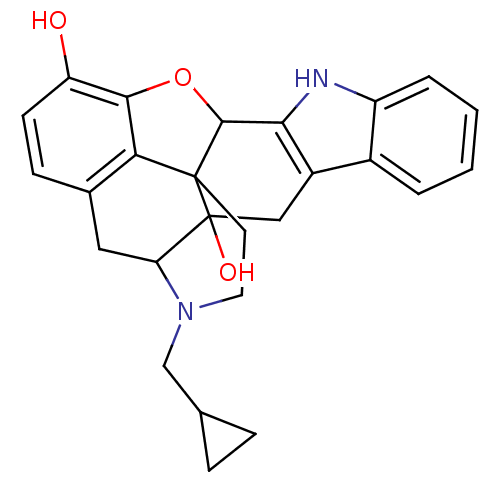 NALTRINDOLE NSC_3034754 BDBM86662 CAS_111555-53-4
NALTRINDOLE NSC_3034754 BDBM86662 CAS_111555-53-4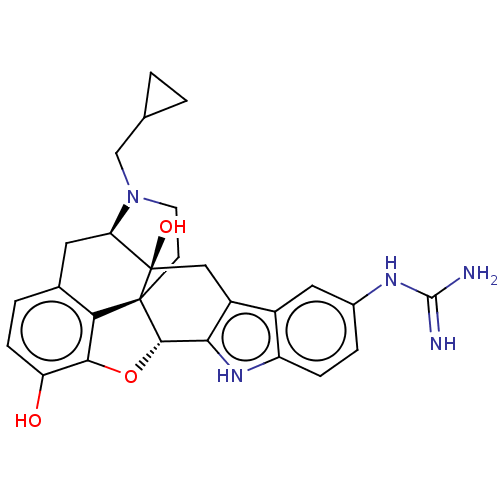 BDBM50027039 5`-Guanidinonaltrindole Guanidinonaltrindole 5''-Guanidinonaltrindole 5''-Guanidinylnaltrindole C5''-Guanidinyl Naltrindole
BDBM50027039 5`-Guanidinonaltrindole Guanidinonaltrindole 5''-Guanidinonaltrindole 5''-Guanidinylnaltrindole C5''-Guanidinyl Naltrindole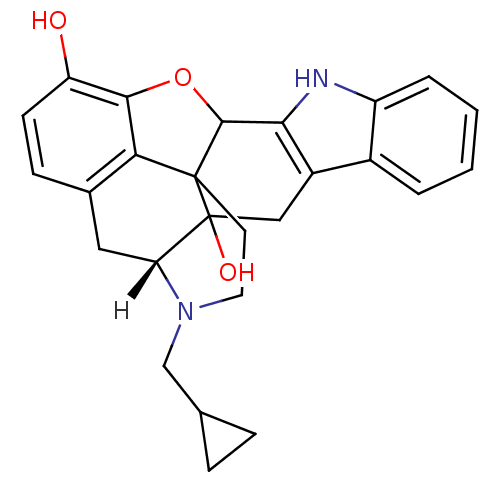 NALTRINDOLE [3H]-naltrindole BDBM21864 (21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5,7,9,15,17,19(25)-heptaene-2,16-diol (8R)-7-(cyclopropylmethyl)-5,6,7,8,14,14b-hexahydro-4,8-methano[1]benzofuro[2,3-a]pyrido[4,3-b]carbazole-1,8a(9H)-diol
NALTRINDOLE [3H]-naltrindole BDBM21864 (21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5,7,9,15,17,19(25)-heptaene-2,16-diol (8R)-7-(cyclopropylmethyl)-5,6,7,8,14,14b-hexahydro-4,8-methano[1]benzofuro[2,3-a]pyrido[4,3-b]carbazole-1,8a(9H)-diol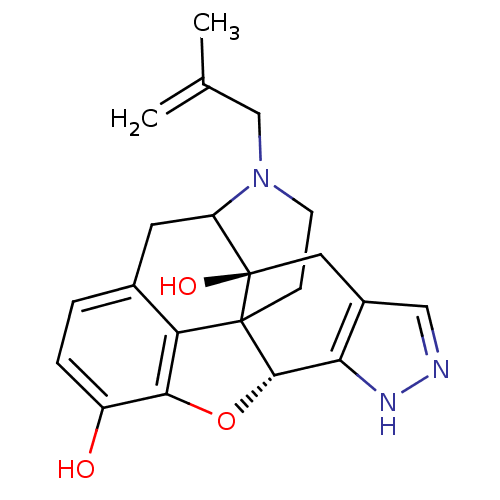 Naltrindole analogue BDBM50105782 18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaene-2,12-diol CHEMBL316930
Naltrindole analogue BDBM50105782 18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaene-2,12-diol CHEMBL316930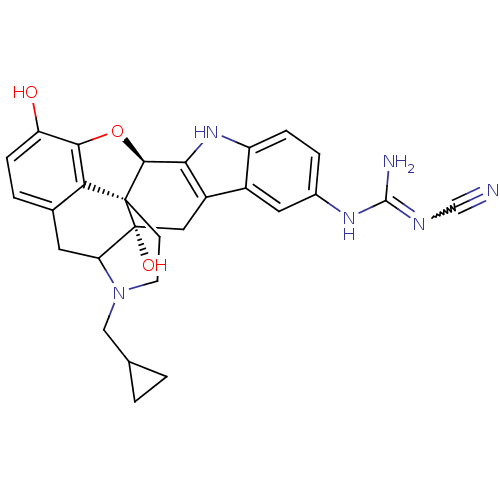 5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6,7-didehydro-4,5alpha-epoxy-3,14-dihydroxyindolo[2',3':6,7]morphinan cyno-C5'-guanidinyl naltrindole BDBM50068379 CHEMBL330751
5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6,7-didehydro-4,5alpha-epoxy-3,14-dihydroxyindolo[2',3':6,7]morphinan cyno-C5'-guanidinyl naltrindole BDBM50068379 CHEMBL330751 12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaen-2-ol Naltrindole analogue BDBM50105778 CHEMBL95847
12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaen-2-ol Naltrindole analogue BDBM50105778 CHEMBL95847 BDBM50121334 Naltrindole analogue 2-ethoxy-13,22-dimethyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol
BDBM50121334 Naltrindole analogue 2-ethoxy-13,22-dimethyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol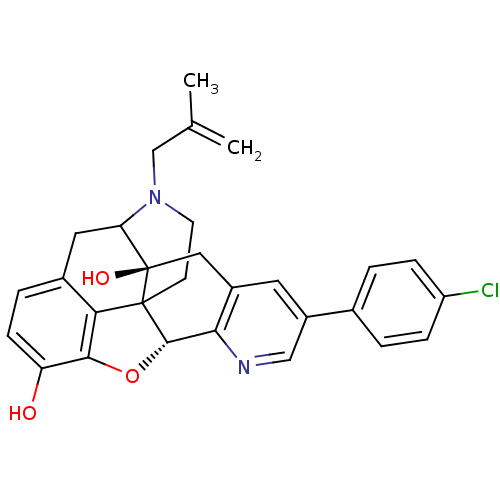 BDBM50105772 Naltrindole analogue CHEMBL317536 6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaene-2,13-diol
BDBM50105772 Naltrindole analogue CHEMBL317536 6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaene-2,13-diol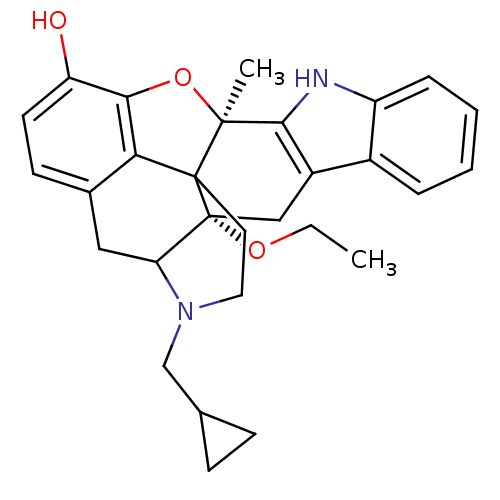 BDBM50121331 Naltrindole analogue 22-cyclopropylmethyl-2-ethoxy-13-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol
BDBM50121331 Naltrindole analogue 22-cyclopropylmethyl-2-ethoxy-13-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol CHEMBL318957 Naltrindole analogue BDBM50105771 6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaen-2-ol
CHEMBL318957 Naltrindole analogue BDBM50105771 6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaen-2-ol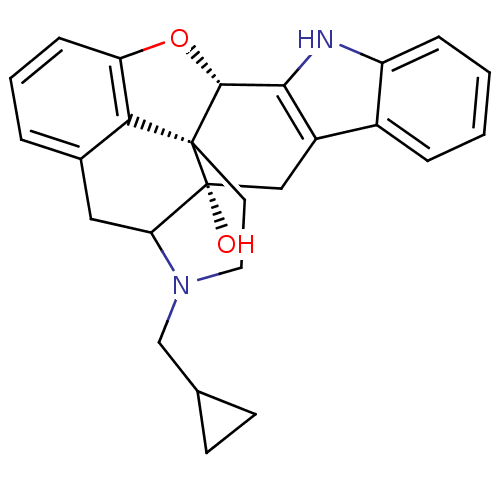 BDBM50291697 Naltrindole analogue (1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-2-ol
BDBM50291697 Naltrindole analogue (1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-2-ol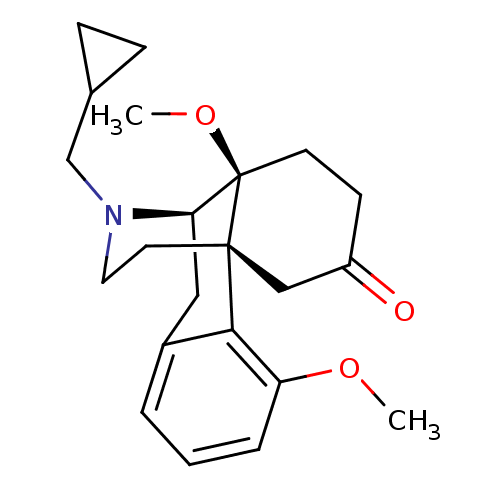 CYPRODIME BROMIDE BDBM50148071 cyprodime Naltrindole analogue 17-cyclopropylmethyl-3,10-dimethoxy-(1R,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-trien-13-one 17-cyclopropylmethyl-10-hydroxy-4,14-dimethoxy-(1S,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3-dien-6-one(cyprodime) (-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6-one(Cyprodime) CHEMBL322796
CYPRODIME BROMIDE BDBM50148071 cyprodime Naltrindole analogue 17-cyclopropylmethyl-3,10-dimethoxy-(1R,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-trien-13-one 17-cyclopropylmethyl-10-hydroxy-4,14-dimethoxy-(1S,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3-dien-6-one(cyprodime) (-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6-one(Cyprodime) CHEMBL322796
- Coop, A; Pinto, J; Wang, L; McCullough, K; Rothman, RB; Dersch, C; Jacobson, AE; Rice, KC Delta opioid binding selectivity of 3-ether analogs of naltrindole. Bioorg Med Chem Lett 9: 3435-8 (2000)
- Portoghese, PS; Larson, DL; Sultana, M; Takemori, AE Opioid agonist and antagonist activities of morphindoles related to naltrindole. J Med Chem 35: 4325-9 (1992)
- Coop, A; Jacobson, AE; Aceto, MD; Harris, LS; Traynor, JR; Woods, JH; Rice, KC N-Cyclohexylethyl-N-noroxymorphindole: a mu-opioid preferring analogue of naltrindole. Bioorg Med Chem Lett 10: 2449-51 (2001)
- Clayson, J; Jales, A; Tyacke, RJ; Hudson, AL; Nutt, DJ; Lewis, JW; Husbands, SM Selective delta-opioid receptor ligands: potential PET ligands based on naltrindole. Bioorg Med Chem Lett 11: 939-43 (2001)
- Sakami, S; Maeda, M; Kawai, K; Aoki, T; Kawamura, K; Fujii, H; Hasebe, K; Nakajima, M; Endo, T; Ueno, S; Ito, T; Kamei, J; Nagase, H Structure-antitussive activity relationships of naltrindole derivatives. Identification of novel and potent antitussive agents. J Med Chem 51: 4404-11 (2008)
- Coop, A; Rothman, RB; Dersch, C; Partilla, J; Porreca, F; Davis, P; Jacobson, AE; Rice, KC delta Opioid affinity and selectivity of 4-hydroxy-3-methoxyindolomorphinan analogues related to naltrindole. J Med Chem 42: 1673-9 (1999)
- Nemoto, T; Iihara, Y; Hirayama, S; Iwai, T; Higashi, E; Fujii, H; Nagase, H Naltrindole derivatives with fluorinated ethyl substituents on the 17-nitrogen asd opioid receptor inverse agonists. Bioorg Med Chem Lett 25: 2927-30 (2015)
- Portoghese, PS; Nagase, H; MaloneyHuss, KE; Lin, CE; Takemori, AE Role of spacer and address components in peptidomimetic delta opioid receptor antagonists related to naltrindole. J Med Chem 34: 1715-20 (1991)
- Ananthan, S; Johnson, CA; Carter, RL; Clayton, SD; Rice, KC; Xu, H; Davis, P; Porreca, F; Rothman, RB Synthesis, opioid receptor binding, and bioassay of naltrindole analogues substituted in the indolic benzene moiety. J Med Chem 41: 2872-81 (1998)
- Portoghese, PS; Ohkawa, S; Moe, ST; Takemori, AE Synthesis and delta-opioid receptor antagonist activity of a naltrindole analogue with a regioisomeric indole moiety. J Med Chem 37: 1886-8 (1994)
- Le Bourdonnec, B; El Kouhen, R; Poda, G; Law, PY; Loh, HH; Ferguson, DM; Portoghese, PS Covalently induced activation of the delta opioid receptor by a fluorogenic affinity label, 7'-(phthalaldehydecarboxamido)naltrindole (PNTI). J Med Chem 44: 1017-20 (2001)
- Jales, AR; Husbands, SM; Lewis, JW Selective kappa-opioid antagonists related to naltrindole. Effect of side-chain spacer in the 5'-amidinoalkyl series. Bioorg Med Chem Lett 10: 2259-61 (2001)
- Portoghese, PS; Sultana, M; Nelson, WL; Klein, P; Takemori, AE Delta opioid antagonist activity and binding studies of regioisomeric isothiocyanate derivatives of naltrindole: evidence for delta receptor subtypes. J Med Chem 35: 4086-91 (1992)
- Duval, RA; Allmon, RL; Lever, JR Indium-labeled macrocyclic conjugates of naltrindole: high-affinity radioligands for in vivo studies of peripheral delta opioid receptors. J Med Chem 50: 2144-56 (2007)
- Black, SL; Jales, AR; Brandt, W; Lewis, JW; Husbands, SM The role of the side chain in determining relative delta- and kappa-affinity in C5'-substituted analogues of naltrindole. J Med Chem 46: 314-7 (2003)
- Portoghese, PS; Farouz-Grant, F; Sultana, M; Takemori, AE 7'-Substituted amino acid conjugates of naltrindole. Hydrophilic groups as determinants of selective antagonism of delta 1 opioid receptor-mediated antinociception in mice. J Med Chem 38: 402-7 (1995)
- Klein, P; Nelson, WL O3-(2-carbomethoxyallyl) ethers of opioid ligands derived from oxymorphone, naltrexone, etorphine, diprenorphine, norbinaltorphimine, and naltrindole. Unexpected O3-dealkylation in the opioid radioligand displacement assay. J Med Chem 35: 4589-94 (1993)
- Sharma, SK; Jones, RM; Metzger, TG; Ferguson, DM; Portoghese, PS Transformation of a kappa-opioid receptor antagonist to a kappa-agonist by transfer of a guanidinium group from the 5'- to 6'-position of naltrindole. J Med Chem 44: 2073-9 (2001)
- Olmsted, SL; Takemori, AE; Portoghese, PS A remarkable change of opioid receptor selectivity on the attachment of a peptidomimetic kappa address element to the delta antagonist, natrindole: 5'-[N2-alkylamidino)methyl]naltrindole derivatives as a novel class of kappa opioid receptor antagonists. J Med Chem 36: 179-80 (1993)
- Ray2010 Assay 14 3H-Naltrindole cloned PDSP; [18].
- ChEMBL_1762410 (CHEMBL4197657) Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes
- ChEMBL_1762418 (CHEMBL4197665) Displacement of [3H]naltrindole from DOR in monkey brain cortex membranes
- ChEMBL_303251 (CHEMBL826377) Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1
- ChEMBL_453748 (CHEMBL902954) Displacement of [3H]naltrindole from human DOP receptor expressed in deltaC6 cells
- ChEMBL_458379 (CHEMBL941713) Displacement of [3H]naltrindole from delta opioid receptor expressed in CHO cells
- ChEMBL_462212 (CHEMBL945981) Displacement of [3H]naltrindole from delta opioid receptor in rat brain membranes
- ChEBML_145496 Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 in CHO cells
- ChEBML_147148 Binding affinity was determined towards Opioid receptor delta 1 using [3H]naltrindole as radioligand
- ChEBML_147167 Displacement of [3H]naltrindole from Opioid receptor delta 1 of guinea pig brain membranes
- ChEMBL_145391 (CHEMBL750081) Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1
- ChEMBL_1762413 (CHEMBL4197660) Displacement of [3H]naltrindole from DOR (unknown origin) expressed in CHO cell membranes
- ChEMBL_330098 (CHEMBL858980) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_392922 (CHEMBL870416) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_393078 (CHEMBL870429) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_434330 (CHEMBL917911) Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells
- ChEMBL_436576 (CHEMBL904884) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane
- ChEMBL_444081 (CHEMBL893244) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane
- ChEMBL_471609 (CHEMBL941038) Displacement of [3H]naltrindole from human delta opioid receptor expressed in deltaC6 cells
- ChEMBL_533285 (CHEMBL973304) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_540501 (CHEMBL1030605) Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_558748 (CHEMBL1019877) Displacement of [3H]Naltrindole form human delta opioid receptor expressed in CHO cells
- ChEMBL_580496 (CHEMBL1052523) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_596275 (CHEMBL1050691) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells
- ChEMBL_622964 (CHEMBL1112216) Displacement of [3H]naltrindole from human delta opioid receptor expressed in deltaC6 cells
- ChEMBL_1552643 (CHEMBL3762582) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cell membrane
- ChEMBL_331365 (CHEMBL867090) Displacement of [3H]naltrindole from cloned human delta opioid receptor expressed in CHO cells
- ChEMBL_438284 (CHEMBL887390) Displacement of [3H]naltrindole from human delta opioid receptors expressed in CHO cell membrane
- ChEMBL_556070 (CHEMBL953423) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cell membrane
- ChEMBL_572572 (CHEMBL1025188) Displacement of [3H]naltrindole from human cloned delta opioid receptor expressed in CHO cells
- ChEMBL_582245 (CHEMBL1060813) Displacement of [3H]naltrindole from human cloned delta opioid receptor expressed in CHO cells
- ChEMBL_145190 (CHEMBL754435) Inhibition of [3H]naltrindole binding to opioid receptor delta 1 of Chinese hamster ovary membrane
- ChEMBL_439350 (CHEMBL888461) Ratio of IC50 of drug to naltrindole for delta opioid receptor in mouse vas deferens
- ChEMBL_467788 (CHEMBL931352) Displacement of [3H]naltrindole from human cloned delta opioid receptor expressed in CHO cell membrane
- ChEBML_147151 Binding affinity towards Opioid receptor delta 1 in guinea pig brain membranes using [3H]naltrindole as radioligand
- ChEMBL_1275491 (CHEMBL3090461) Displacement of [3H]-naltrindole from human delta opioid receptor transfected in CHO cells after 3 hrs
- ChEMBL_147143 (CHEMBL754175) Binding affinity to displace radioligand [3H]naltrindole on Opioid receptor delta 1 in guinea pig membranes.
- ChEMBL_147150 (CHEMBL758175) Binding affinity towards Opioid receptor delta 1 from guinea pig brain using [3H]naltrindole as radioligand
- ChEMBL_147194 (CHEMBL756870) The binding affinity was measured using [3H]naltrindole against Opioid receptor delta 1 of rat brain
- ChEMBL_303256 (CHEMBL826382) Binding affinity against Opioid receptor delta 1 expressed in CHO cells using [3H]Naltrindole as radioligand
- ChEMBL_980540 (CHEMBL2422699) Displacement of [3H]-naltrindole from mouse delta opioid receptor expressed in CHO cells after 1.5 hrs
- ChEMBL_145334 (CHEMBL750422) Binding inhibition of [3H]- -naltrindole (0.15 nM) to membranes in CHO cells expressing Opioid receptor delta 1
- ChEMBL_147025 (CHEMBL753666) Binding affinity against opioid receptor delta 1 using [3H]naltrindole as radioligand in guinea pig brain membranes.
- ChEMBL_147151 (CHEMBL758176) Binding affinity towards Opioid receptor delta 1 in guinea pig brain membranes using [3H]naltrindole as radioligand
- ChEMBL_145335 (CHEMBL750570) Inhibition of binding of [3H]- -naltrindole (0.15 nM) to membranes from CHO cells expressing Opioid receptor delta 1
- ChEMBL_145608 (CHEMBL749751) Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells.
- ChEMBL_147149 (CHEMBL758174) Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane
- ChEMBL_1762407 (CHEMBL4197654) Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting method
- ChEBML_145618 Tested for the opioid receptor delta 1 binding affinity using membrane preparations from recombinant HEK293 cells with [3H]naltrindole radioligand
- ChEBML_147147 Binding affinity was determined against Opioid receptor delta 1 obtained from guinea pig brain membranes using [3H]naltrindole as radioligand
- ChEMBL_146320 (CHEMBL757529) Binding affinity against Opioid receptor delta 1 receptor binding in ICR mouse brain membranes using the radioligand [3H]naltrindole
- ChEMBL_1465625 (CHEMBL3405073) Displacement of [3H]naltrindole from mouse DOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay
- ChEMBL_564843 (CHEMBL955070) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting
- ChEMBL_717378 (CHEMBL1670100) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting
- ChEMBL_740229 (CHEMBL1764074) Displacement of [3H]-naltrindole from human mu opioid receptor expressed in CHO cells after 2 hrs by scintillation counting
- ChEMBL_742587 (CHEMBL1769430) Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting
- ChEMBL_746789 (CHEMBL1777019) Displacement of [3H]naltrindole from human delta-opioid receptor expressed in CHO cells after 3 hrs by scintillation counting
- ChEMBL_827236 (CHEMBL2050635) Displacement of [3H]-naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting
- ChEMBL_887195 (CHEMBL2215303) Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting
- ChEMBL_145615 (CHEMBL754334) Inhibitory activity against Opioid receptor delta 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]naltrindole radioligand
- ChEMBL_147147 (CHEMBL754179) Binding affinity was determined against Opioid receptor delta 1 obtained from guinea pig brain membranes using [3H]naltrindole as radioligand
- ChEMBL_1507462 (CHEMBL3599487) Displacement of [3H]-naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting analysis
- ChEMBL_1615868 (CHEMBL3857937) Displacement of [3H]naltrindole from human delta-opioid receptor expressed in CHO cell membranes after 3 hrs by scintillation counting
- ChEMBL_591236 (CHEMBL1056141) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting
- ChEMBL_616851 (CHEMBL1100235) Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cell membranes after 3 hrs by scintillation counting
- ChEMBL_740153 (CHEMBL1763213) Displacement of [3H]-naltrindole from human kappa opioid receptor expressed in human HEK293 cells after 2 hrs by scintillation counting
- ChEBML_217734 Compound was tested for inhibition of binding of [3H]- -naltrindole (0.15 nM) to membranes from CHO cells expressing human delta opioid receptor
- ChEBML_217735 Compound was tested for inhibition of binding of [3H]- -naltrindole (0.15 nM) to membranes from CHO cells expressing human delta opioid receptor
- ChEMBL_591965 (CHEMBL1047528) Displacement of [3H]naltrindole from human cloned delta opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting
- ChEMBL_145884 (CHEMBL754250) In vitro antagonist activity against Opioid receptor delta 1 in the presence of 30 nM naltrindole, in a mouse vas deferens assay
- ChEMBL_145903 (CHEMBL750392) Inv itro antagonist activity against Opioid receptor delta 1 in the presence of 30 nM naltrindole, in a mouse vas deferens assay
- ChEMBL_1825158 (CHEMBL4324922) Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay
- ChEMBL_217734 (CHEMBL823798) Compound was tested for inhibition of binding of [3H]- -naltrindole (0.15 nM) to membranes from CHO cells expressing human delta opioid receptor
- ChEMBL_217735 (CHEMBL823799) Compound was tested for inhibition of binding of [3H]- -naltrindole (0.15 nM) to membranes from CHO cells expressing human delta opioid receptor
- ChEBML_147161 Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]naltrindole as radioligand
- Competitive Displacement Assay Membrane protein from CHO (Chinese Hamster Ovarian) cells that stably expressed one type of the cloned human opioid receptor were incubated with 12 different concentrations of the compound in the presence of 0.25 nM [3H]DAMGO, 0.2 nM [3H]naltrindole or 1 nM [3H]U69,593 in a final volume of 1 mL of 50 mM Tris-HCl, pH 7.5 at 25° C. Incubation times of 60 min were used for [3H]DAMGO and [3H]U69,593. Because of a slower association of [3H]naltrindole with the receptor, a 3 h incubation was used with this radioligand. Samples incubated with [3H]naltrindole also contained 10 mM MgCl2 and 0.5 mM phenylmethylsulfonyl fluoride. Nonspecific binding was measured by inclusion of 10 μM naloxone. The binding was terminated by filtering the samples through Schleicher & Schuell No. 32 glass fiber filters using a Brandel 48-well cell harvester. The filters were subsequently washed three times with 3 mL of cold 50 mM Tris-HCl, pH 7.5, and were counted in 2 mL Ecoscint A scintillation fluid. For [3H]naltrindole and [3H]U69,593 binding, the filters were soaked in 0.1% polyethylenimine for at least 60 min before use.
- Competitive Displacement Assay Receptor Binding (in vitro Assay) The Ki (binding affinity) for mu-, delta-, and kippa-receptors was determined with a previously described method using a competitive displacement assay (Neumeyer, 2003). Membrane protein from CHO (Chinese Hamster Ovarian) cells that stably expressed one type of the cloned human opioid receptor were incubated with 12 different concentrations of the compound in the presence of 0.25 nM [3H]DAMGO, 0.2 nM [3H]naltrindole or 1 nM [3H]U69,593 in a final volume of 1 mL of 50 mM Tris-HCl, pH 7.5 at 25 C. Incubation times of 60 min were used for [3H]DAMGO and [3H]U69,593. Because of a slower association of [3H]naltrindole with the receptor, a 3 h incubation was used with this radioligand. Samples incubated with [3H]naltrindole also contained 10 mM MgCl2 and 0.5 mM phenylmethylsulfonyl fluoride. Nonspecific binding was measured by inclusion of 10 uM naloxone. The binding was terminated by filtering the samples through Schleicher & Schuell No. 32 glass.
- Receptor Binding Assay The Ki (binding affinity) for μ-receptor was determined with a previously described method using a competitive displacement assay (Neumeyer et al., J. Med. Chem., v. 46, p. 5162-5170, 2003). Membrane protein from CHO (Chinese Hamster Ovarian) cells that stably expressed one type of the cloned human opioid receptor were incubated with 12 different concentrations of the compound in the presence of 0.25 nM [3H]DAMGO, 0.2 nM [3H]naltrindole or 1 nM [3H]U69,593 in a final volume of 1 mL of 50 mM Tris-HCl, pH 7.5 at 25° C. Incubation times of 60 min were used for [3H]DAMGO and [3H]U69,593. Because of a slower association of [3H]naltrindole with the receptor, a 3 h incubation was used with this radioligand. Samples incubated with [3H]naltrindole also contained 10 mM MgCl2 and 0.5 mM phenylmethylsulfonyl fluoride. Nonspecific binding was measured by inclusion of 10 μM naloxone. The binding was terminated by filtering the samples through Schleicher & Schuell No. 32 glass fiber filters using a Brandel 48-well cell harvester. The filters were subsequently washed three times with 3 mL of cold 50 mM Tris-HCl, pH 7.5, and were counted in 2 mL Ecoscint A scintillation fluid. For [3H]naltrindole and [3H]U69,593 binding, the filters were soaked in 0.1% polyethylenimine for at least 60 min before use. IC50 values will be calculated by least squares fit to a logarithm-probit analysis. Ki values of unlabelled compounds were calculated from the equation Ki=(IC50)/1+S where S=(concentration of radioligand)/(Kd of radioligand).
- δ-Opioid Receptor Binding Assays δ-Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid receptor expressend in CHO-K1 cells; Perkin Elmer) in a final volume of 5004 binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25μm M unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25° C.
- Beta-Opioid Binding Assay Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 ug membrane protein (recombinant delta opioid receptor expressend in CHO-K1 cells; Perkin Elmer) in a final volume of 500 uL binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 uM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25 °C. Binding reactions were determined by rapid filtration onto 96-well Unifilter GF/C filter plates (Packard) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Packard) followed by five filtration washes with 500 uL ice-cold binding buffer. Filter plates were subsequently dried at 50 °C. for 1-2 hours. Fifty uL/well scintillation cocktail (MicroScint20, Packard) was added.
- Binding Assay Delta-Opioid Receptor Binding Assay Procedures: delta-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin Elmer, Shelton, Conn.; 33.0 Ci/mmole) with 5 ug membrane protein (Perkin Elmer, Shelton, Conn.) in a final volume of 500 ul binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 uM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 hr at a temperature of about 25 C. Binding reactions were terminated by rapid filtration onto 96-well Unifilter GF/C filter plates (Perkin Elmer, Shelton, Conn.) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Perkin Elmer, Shelton, Conn.) followed by five filtration washes with 500 ul ice-cold binding buffer. Filter plates were subsequently dried at 50 C. for 1-2 hours.
- Radioligand Binding Assay Radioligand dose-displacement assays used 0.2nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20ug membrane protein (recombinant delta opioid receptor expressend in CHO-K1 cells; Perkin Elmer) in a final volume of 500uL binding buffer (5mM MgCl2, 5% DMSO, 50mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 uM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25C. Binding reactions were determined by rapid filtration onto 96-well Unifilter GF/C filter plates (Packard) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Packard) followed by five filtration washes with 500uL ice-cold binding buffer. Filter plates were subsequently dried at 50C for 1-2 hours. Fifty uL/well scintillation cocktail (MicroScint20, Packard) was added and plates were counted in a Packard Top-Count for 1 min/well.
- Radioligand Dose-Displacement Binding Assay δ-Opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (Perkin Elmer, Shelton, Conn.; 33.0 Ci/mmole) with 5 μg membrane protein (Perkin Elmer, Shelton, Conn.) in a final volume of 500 μl binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 hour at a temperature of about 25° C. Binding reactions were terminated by rapid filtration onto 96-well Unifilter GF/C filter plates (Perkin Elmer, Shelton, Conn.) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Perkin Elmer, Shelton, Conn.) followed by five filtration washes with 500 μl ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hours.
- Radioligand Dose-Displacement Binding Assay δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin Elmer, Shelton, Conn.; 33.0 Ci/mmole) with 5 μg membrane protein (Perkin Elmer, Shelton, Conn.) in a final volume of 500 μl binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 hr at a temperature of about 25° C. Binding reactions were terminated by rapid filtration onto 96-well Unifilter GF/C filter plates (Perkin Elmer, Shelton, Conn.) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Perkin Elmer, Shelton, Conn.) followed by five filtration washes with 500 μl ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hours.
- Radioligand Dose-Displacement Binding Assay Delta-Opioid Receptor Binding Assay Procedures: delta-Opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin Elmer, Shelton, Conn.; 33.0 Ci/mmole) with 5 ug membrane protein (Perkin Elmer, Shelton, Conn.) in a final volume of 500 ul binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 uM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 hour at a temperature of about 25 C. Binding reactions were terminated by rapid filtration onto 96-well Unifilter GF/C filter plates (Perkin Elmer, Shelton, Conn.) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Perkin Elmer, Shelton, Conn.) followed by five filtration washes with 500 ul ice-cold binding buffer. Filter plates were subsequently dried at 50 C. for 1-2 hours.
- Radioligand Dose-Displacement Binding Assay Delta-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin Elmer, Shelton, Conn.; 33.0 Ci/mmole) with 5 ug membrane protein (Perkin Elmer, Shelton, Conn.) in a final volume of 500 ul binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 uM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 hr at a temperature of about 25° C. Binding reactions were terminated by rapid filtration onto 96-well Unifilter GF/C filter plates (Perkin Elmer, Shelton, Conn.) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Perkin Elmer, Shelton, Conn.) followed by five filtration washes with 500 ul ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hours.
- In Vitro Delta-Opioid Receptor Binding Assay δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid receptor expressed in CHO-K1 cells; Perkin Elmer) in a final volume of 500 μL binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25° C. Binding reactions were determined by rapid filtration onto 96-well Unifilter GF/C filter plates (Packard) presoaked in 0.5% polyethylenimine (Sigma-Aldrich). Harvesting was performed using a 96-well tissue harvester (Packard) followed by five filtration washes with 500 μL ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hours. Fifty μL/well scintillation cocktail (MicroScint20, Packard) was added and plates were counted in a Packard Top-Count for 1 min/well.
- In Vitro Delta-Opioid Receptor Binding Assay δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid receptor expressed in CHO-K1 cells; Perkin Elmer) in a final volume of 500 μL binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25° C. Binding reactions were determined by rapid filtration onto 96-well Unifilter GF/C filter plates (Packard) presoaked in 0.5% polyethylenimine (Sigma-Aldrich). Harvesting was performed using a 96-well tissue harvester (Packard) followed by five filtration washes with 500 μL ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hrs. Fifty μL/well scintillation cocktail (MicroScint20, Packard) was added and plates were counted in a Packard Top-Count for 1 min/well.
- In Vitro Delta-Opioid Receptor Binding Assay Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid receptor expressed in CHO-K1 cells; Perkin Elmer) in a final volume of 500 μL binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25 °C. Binding reactions were determined by rapid filtration onto 96-well Unifilter GF/C filter plates (Packard) presoaked in 0.5% polyethylenimine (Sigma-Aldrich). Harvesting was performed using a 96-well tissue harvester (Packard) followed by five filtration washes with 500 μL ice-cold binding buffer. Filter plates were subsequently dried at 50 °C. for 1-2 hours. Fifty μL/well scintillation cocktail (MicroScint20, Packard) was added and plates were counted in a Packard Top-Count for 1 min/well.
- Radioligand Binding Assay Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid receptor expressed in CHO-K1 cells; Perkin Elmer) in a final volume of 500 μL binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 h at a temperature of about 25° C. Binding reactions were determined by rapid filtration onto 96-well Unifilter GF/C filter plates (Packard) presoaked in 0.5% polyethylenimine (Sigma-Aldrich). Harvesting was performed using a 96-well tissue harvester (Packard) followed by five filtration washes with 500 μL ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hrs. Fifty μL/well scintillation cocktail (MicroScint20, Packard) was added and plates were counted in a Packard Top-Count for 1 min/well.
- δ-Opioid Receptor Binding Assay δ-Opioid Receptor Binding Assay Procedures: δ-Opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin Elmer, Shelton, Conn.; 33.0 Ci/mmole) with 5 μg membrane protein (Perkin Elmer, Shelton, Conn.) in a final volume of 500 μl binding buffer (5 mM MgCl2, 5% DMSO, 50 mM Trizma base, pH 7.4). Non-specific binding was determined in the presence of 25 μM unlabeled naloxone. All reactions were performed in 96-deep well polypropylene plates for 1 hour at a temperature of about 25° C. Binding reactions were terminated by rapid filtration onto 96-well Unifilter GF/C filter plates (Perkin Elmer, Shelton, Conn.) presoaked in 0.5% polyethylenimine (Sigma). Harvesting was performed using a 96-well tissue harvester (Perkin Elmer, Shelton, Conn.) followed by five filtration washes with 500 μl ice-cold binding buffer. Filter plates were subsequently dried at 50° C. for 1-2 hours. Fifty μl/well scintillation cocktail (Perkin Elmer, Shelton, Conn.) was added and plates are counted in a Packard Top-Count for 1 min/well.δ-Opioid Receptor Binding Data: In certain embodiments, Compounds of the Invention exhibit a Ki (nM) for δ receptors of about 10,000 or more (which, for the purposes of this invention, is interpreted as having no binding to the δ receptors). Certain Compounds of the Invention exhibit a Ki (nM) of about 20,000 or less for δ receptors. In one embodiment, Compounds of the Invention exhibit a Ki (nM) of about 10,000 or less; or of about 9000 or less for δ receptors. In another embodiment, Compounds of the Invention exhibit a Ki (nM) of about 7500 or less; or of about 6500 or less; or of about 5000 or less; or of about 3000 or less; or of about 2500 or less for δ receptors. In another embodiment, Compounds of the Invention exhibit a Ki (nM) of about 1000 or less; or of about 500 or less; or of about 350 or less; or of about 250 or less; or of about 100 or less; or of about 10 or less for δ receptors.
- Opioid Receptor Binding Assay The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (human embryonic kidney cell line) that were heterologously expressed for the recombinant human mu, delta or kappa opioid receptors.The assay buffers used for opioid receptor binding studies were 50 mM Tris.HCl (pH 7.4) for KOR, 50 mM Tris.HCl (pH 7.4) with 5 mM MgCl2 for MOR, and 50 mM Tris.HCl (pH 7.4) with 10 mM MgCl2 plus 1 mM EDTA for DOR. The wash buffer solution contained 50 mM Tris.HCl with pH 7.4.The opioid receptor binding affinity were compared to three known standards: Naltrindole, U-50488 (trans-(+)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]phenylacetamide, see M. Doi, T. Ishida and M, Inoue; Structure of K-agonist, U-50488 Acta Cryst. (1990). C46, 676-678), and DAMGO (D-Ala2 MePhe4,Gly(ol)5]encephalin, see Allan D. Blake, George Bot, John C. Freeman, and Terry Reisine Differential Opioid Agonist Regulation of the Mouse m Opioid Receptor* THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 2, Issue of January 10, pp. 782-790, 1997).The radio ligands were prepared at the final concentration of 0.5 nM for [3H]DAMGO, 0.5 nM for [3H]diprenorphine, and 0.5 nM for [3H] DADLE, which were used as the competing radioligands for mu, kappa and delta receptor respectively.Cell membrane of HEK293 cells transfected with opioid receptors was prepared in the amount of 20 ug of MOR, 6.7 ug of KOR and 6.7 ug of DOR per each well respectively. These membranes containing the receptor of interest were incubated with increasing concentrations of test compound in the presence of a single concentration of radioligand. The fixed concentration of the radioligand was used and serial dilutions of the test compound were prepared.Testing started at 10 uM of testing compound to 4-fold serial dilution for 8-points detection. 1 μl of compounds/high control/low control was transferred in to the 96 well plates according to the plate map, and then 100 μl of membrane stock solution was dispensed into the plate followed by 100 μl of radio ligand solution. The well plated were incubated for 1 hour at room temperature with 300 rpm gentle agitation. Then, soaked the Unifilter-96 GF/C filter plates with 50 μl of 0.3% Poly ethyleneimine per well for at least 0.5 hour at room temperature, and filtered the reaction mixture through the plates using FilterMate harvester, then wash each plate for four times with cold wash buffer. The filter plates are then dried for 1 hour at 50° C. After drying, the filter was sealed in polyethylene and adds 50 μl of Perkin Elmer Microscint 20 cocktail and the radioactivity counted in a Perkin Elmer MicroBeta2 counter.Specific binding is determined by subtraction of the Bound CPM values in the presence of 50-100× excess of cold ligand. Data is fitted using the saturation analysis non-linear curve fitting routines in Prism . Calculation of the inhibition was conducted using following equation: % Inhibition=(1−(Assay well−Average_LC)/(Average_HC−Average_LC))*100%Binding data was analyzed using GraphPad Prism 5.0 and IC50 data was generated by non-linear regression from dose response curves. Use the model log (inhibitor) vs. response Variable slope was used to fit the data.
 BDBM50370067 US12215173, Compound Naltrindole CHEMBL1237164
BDBM50370067 US12215173, Compound Naltrindole CHEMBL1237164 NALTRINDOLE NSC_3034754 BDBM86662 CAS_111555-53-4
NALTRINDOLE NSC_3034754 BDBM86662 CAS_111555-53-4 BDBM50027039 5`-Guanidinonaltrindole Guanidinonaltrindole 5''-Guanidinonaltrindole 5''-Guanidinylnaltrindole C5''-Guanidinyl Naltrindole
BDBM50027039 5`-Guanidinonaltrindole Guanidinonaltrindole 5''-Guanidinonaltrindole 5''-Guanidinylnaltrindole C5''-Guanidinyl Naltrindole NALTRINDOLE [3H]-naltrindole BDBM21864 (21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5,7,9,15,17,19(25)-heptaene-2,16-diol (8R)-7-(cyclopropylmethyl)-5,6,7,8,14,14b-hexahydro-4,8-methano[1]benzofuro[2,3-a]pyrido[4,3-b]carbazole-1,8a(9H)-diol
NALTRINDOLE [3H]-naltrindole BDBM21864 (21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5,7,9,15,17,19(25)-heptaene-2,16-diol (8R)-7-(cyclopropylmethyl)-5,6,7,8,14,14b-hexahydro-4,8-methano[1]benzofuro[2,3-a]pyrido[4,3-b]carbazole-1,8a(9H)-diol Naltrindole analogue BDBM50105782 18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaene-2,12-diol CHEMBL316930
Naltrindole analogue BDBM50105782 18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaene-2,12-diol CHEMBL316930 5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6,7-didehydro-4,5alpha-epoxy-3,14-dihydroxyindolo[2',3':6,7]morphinan cyno-C5'-guanidinyl naltrindole BDBM50068379 CHEMBL330751
5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6,7-didehydro-4,5alpha-epoxy-3,14-dihydroxyindolo[2',3':6,7]morphinan cyno-C5'-guanidinyl naltrindole BDBM50068379 CHEMBL330751 12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaen-2-ol Naltrindole analogue BDBM50105778 CHEMBL95847
12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9.02,17.04,8.015,21]henicosa-4(8),5,11,13,15(21)-pentaen-2-ol Naltrindole analogue BDBM50105778 CHEMBL95847 BDBM50121334 Naltrindole analogue 2-ethoxy-13,22-dimethyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol
BDBM50121334 Naltrindole analogue 2-ethoxy-13,22-dimethyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol BDBM50105772 Naltrindole analogue CHEMBL317536 6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaene-2,13-diol
BDBM50105772 Naltrindole analogue CHEMBL317536 6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaene-2,13-diol BDBM50121331 Naltrindole analogue 22-cyclopropylmethyl-2-ethoxy-13-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol
BDBM50121331 Naltrindole analogue 22-cyclopropylmethyl-2-ethoxy-13-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-16-ol CHEMBL318957 Naltrindole analogue BDBM50105771 6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaen-2-ol
CHEMBL318957 Naltrindole analogue BDBM50105771 6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(2S,10R)-11-oxa-8,19-diazahexacyclo[10.9.1.01,10.02,18.04,9.016,22]docosa-4(9),5,7,12,14,16(22)-hexaen-2-ol BDBM50291697 Naltrindole analogue (1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-2-ol
BDBM50291697 Naltrindole analogue (1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.0^{1,13}.0^{2,21}.0^{4,12}.0^{5,10}.0^{19,25}]pentacosa-4(12),5(10),6,8,15,17,19(25)-heptaen-2-ol CYPRODIME BROMIDE BDBM50148071 cyprodime Naltrindole analogue 17-cyclopropylmethyl-3,10-dimethoxy-(1R,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-trien-13-one 17-cyclopropylmethyl-10-hydroxy-4,14-dimethoxy-(1S,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3-dien-6-one(cyprodime) (-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6-one(Cyprodime) CHEMBL322796
CYPRODIME BROMIDE BDBM50148071 cyprodime Naltrindole analogue 17-cyclopropylmethyl-3,10-dimethoxy-(1R,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-trien-13-one 17-cyclopropylmethyl-10-hydroxy-4,14-dimethoxy-(1S,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3-dien-6-one(cyprodime) (-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6-one(Cyprodime) CHEMBL322796