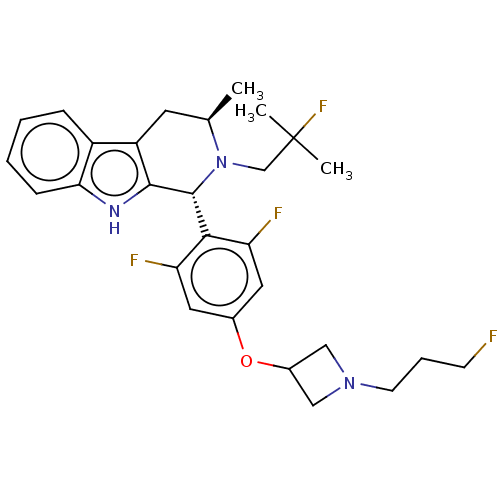
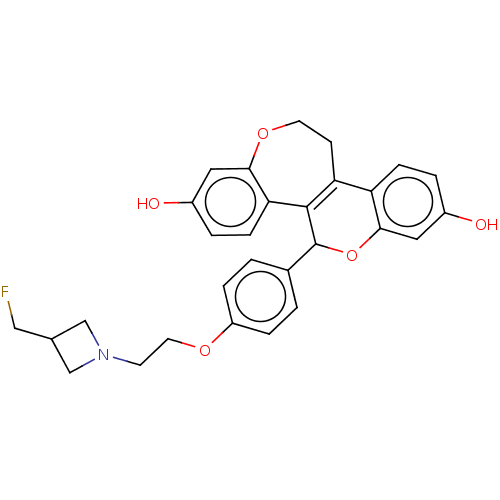
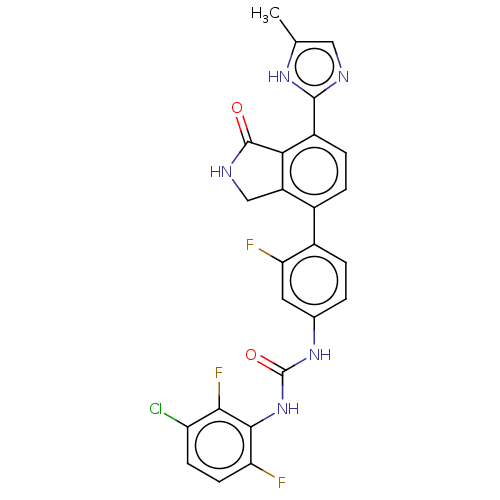
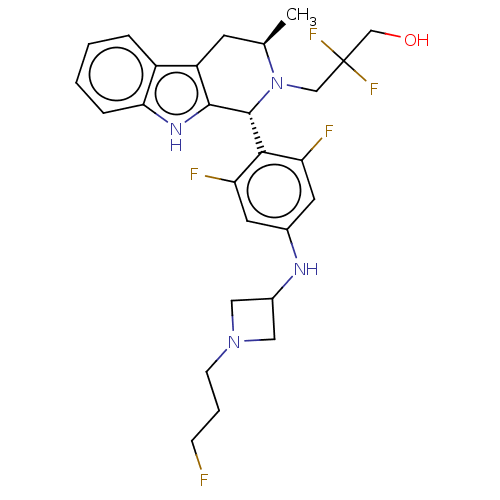
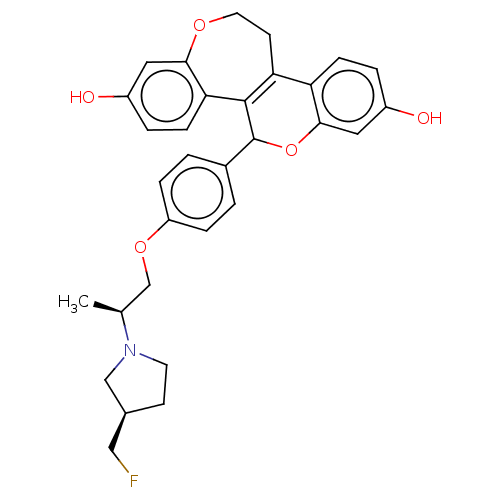
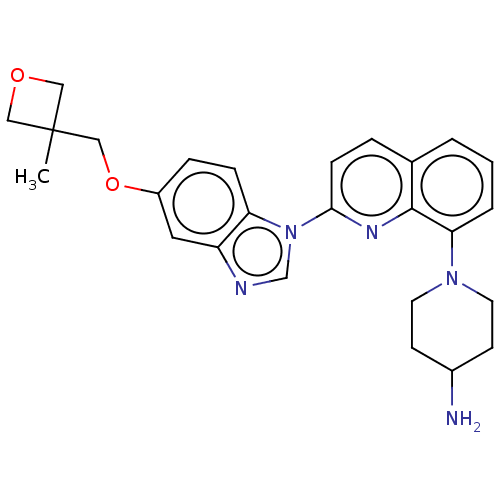
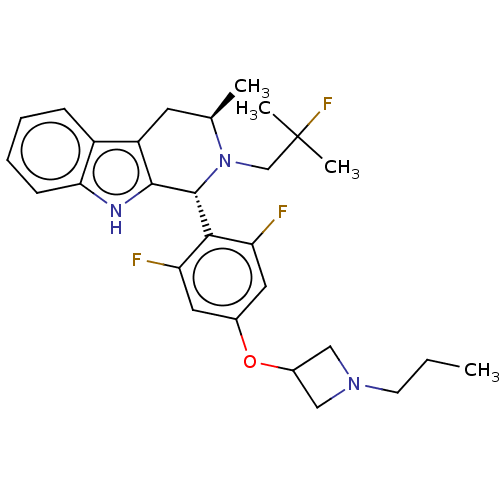
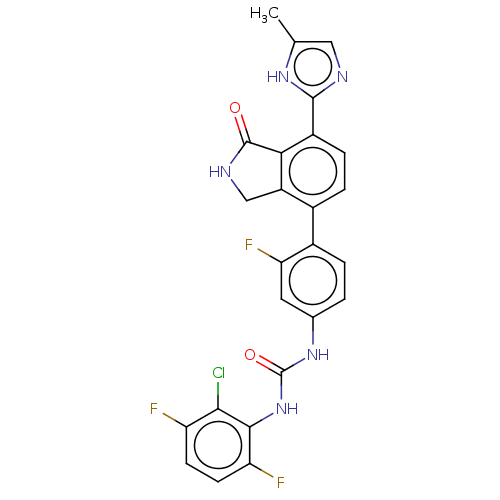
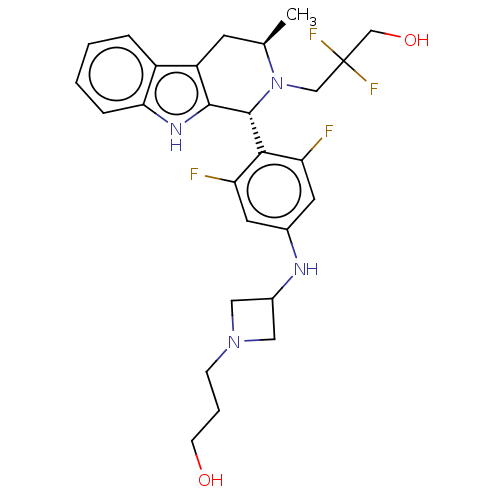
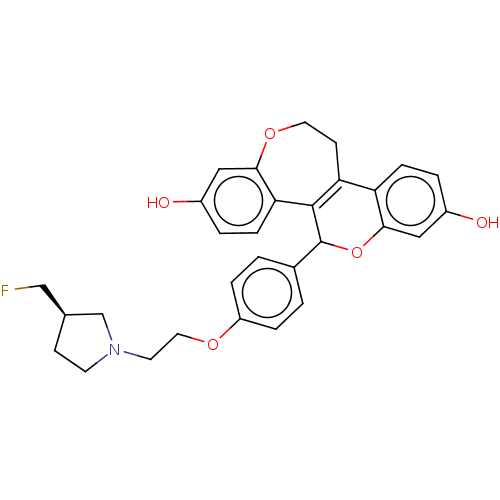
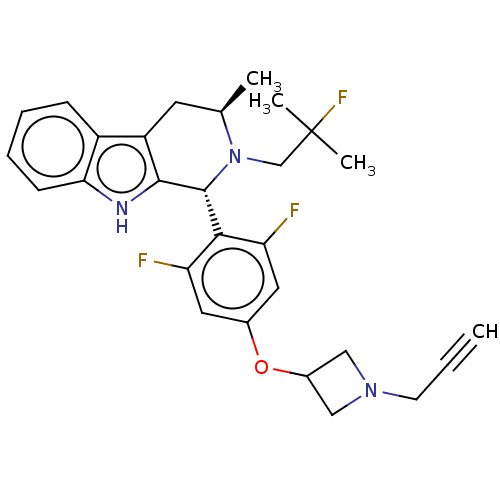
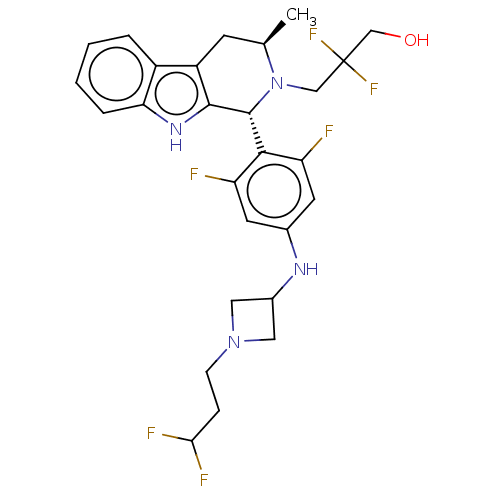
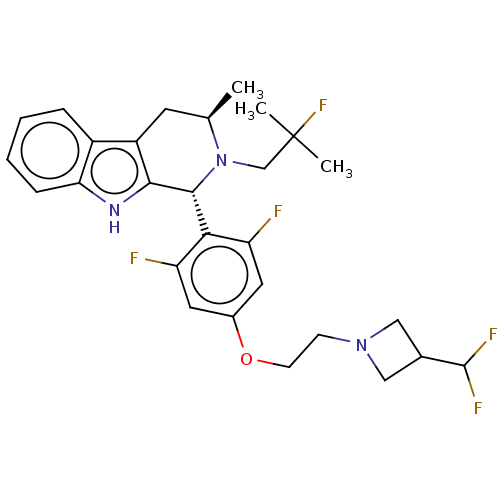
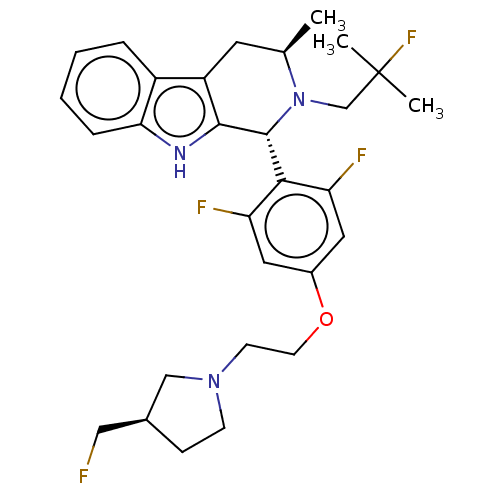
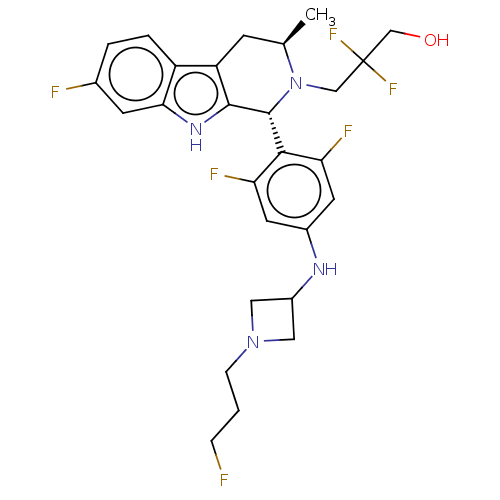
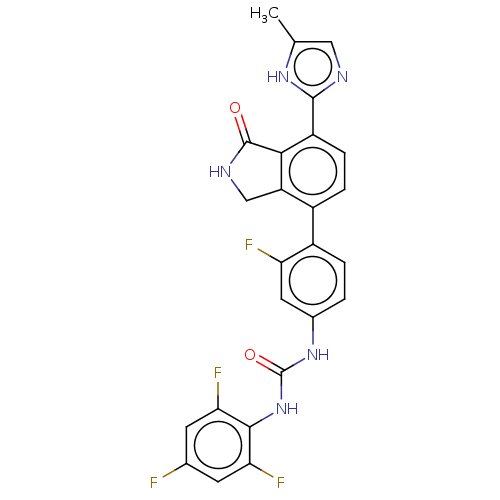
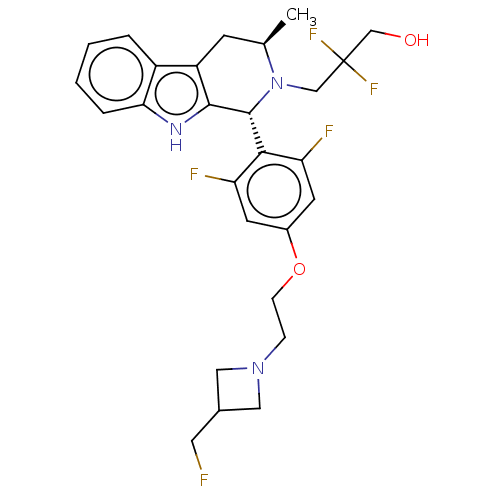
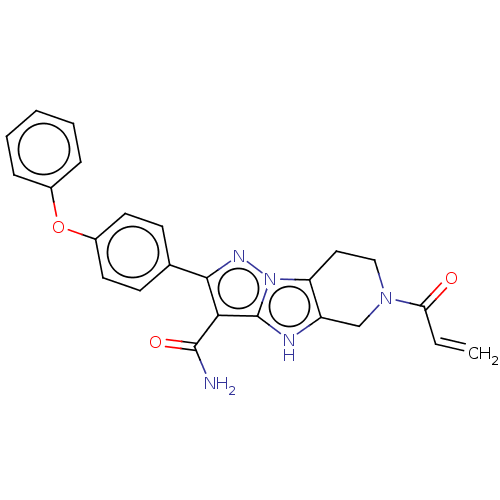
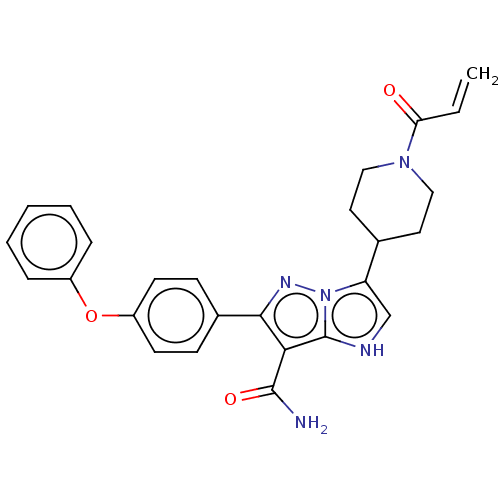
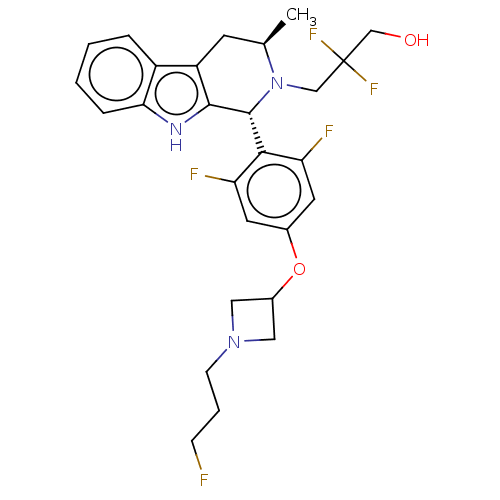
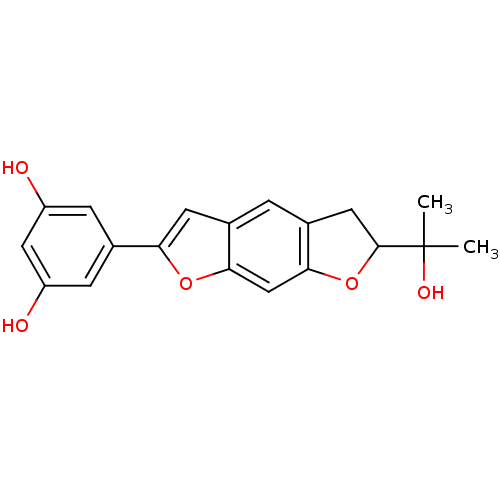
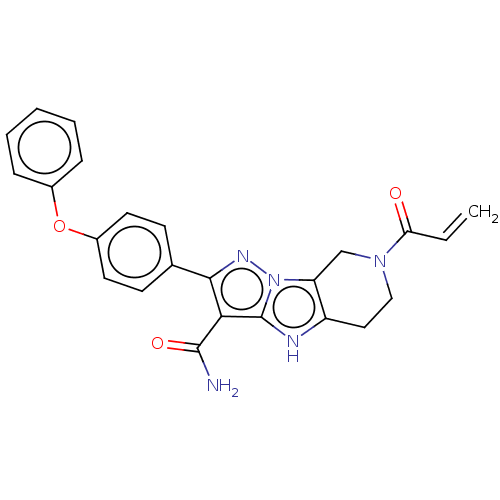
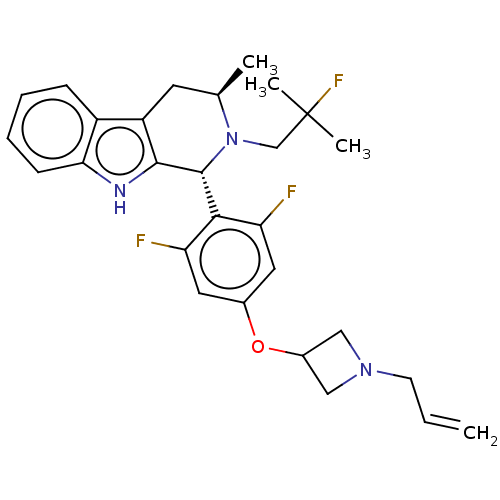
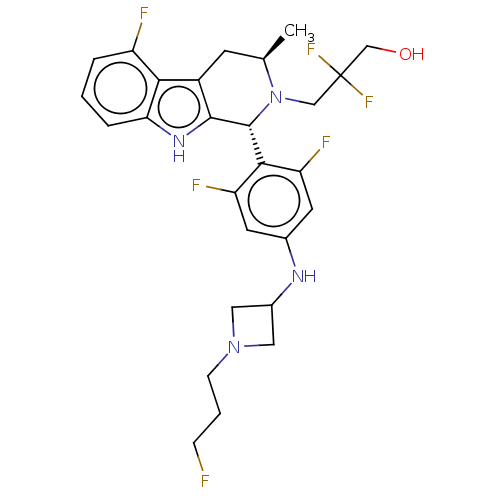
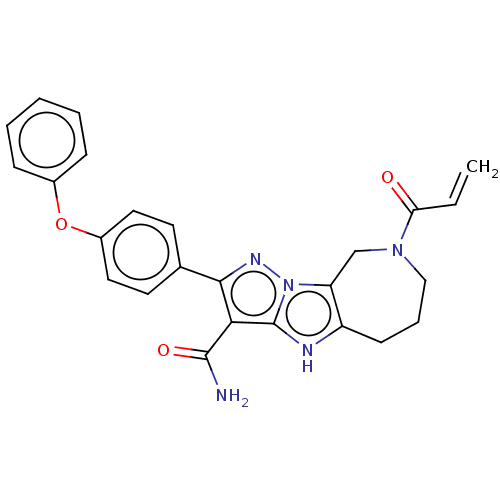
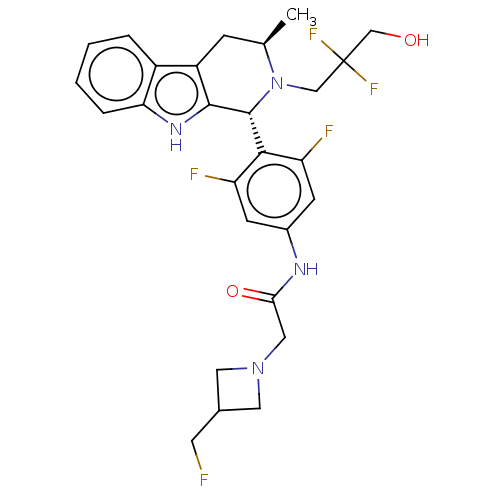
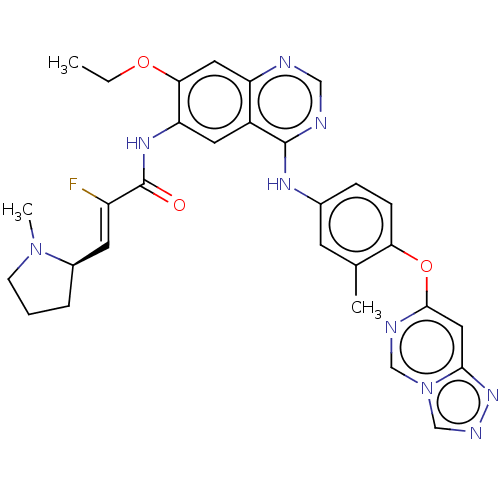
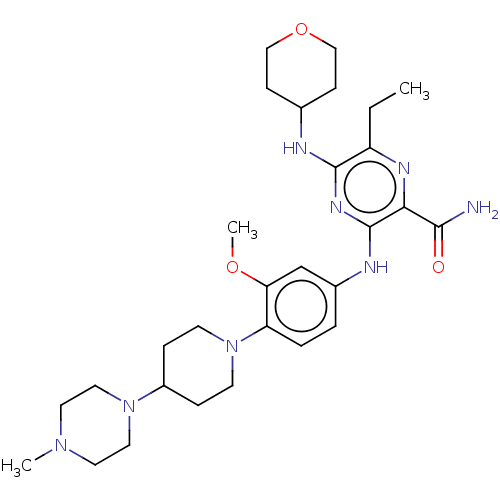
Affinity DataIC50: 0.0240nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0240nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
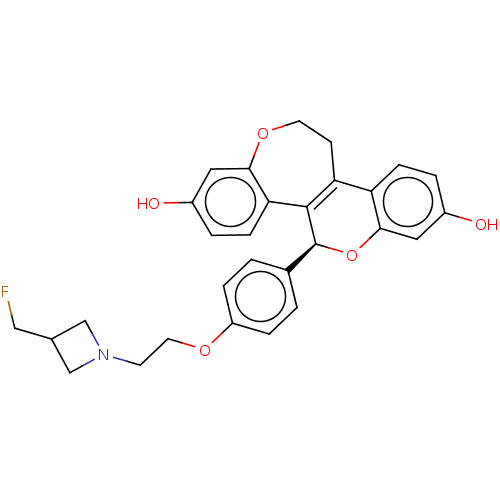
Affinity DataIC50: 0.0300nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
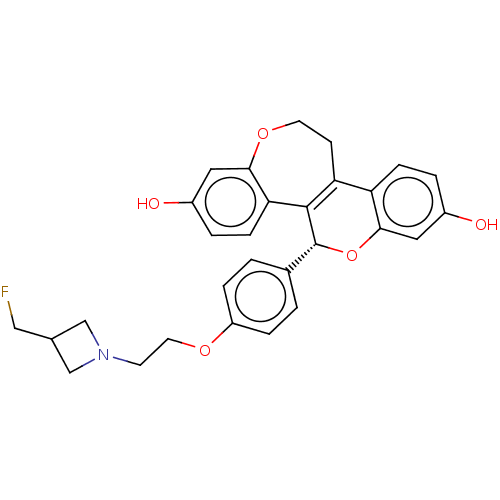
Affinity DataIC50: 0.0310nMAssay Description:Induction of ERalpha degradation in human T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0450nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
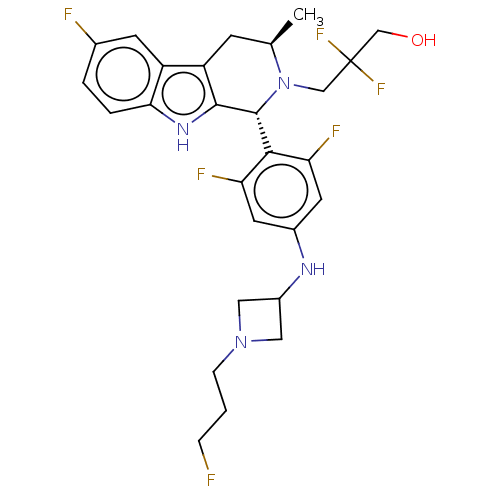
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0510nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0530nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0550nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0590nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of FLT3 D835Y mutant (unknown origin) phosphorylation in mouse BaF3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0650nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0760nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0830nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
TargetHypoxia-inducible factor 1-alpha(Homo sapiens (Human))
Korean Research Institute of Biosciences and Biotechnology
Curated by ChEMBL
Korean Research Institute of Biosciences and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assayMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Shanghai Pharmaceuticals Holding
Curated by ChEMBL
Shanghai Pharmaceuticals Holding
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:Inhibition of HER2 V777L mutant (unknown origin) incubated for 120 mins in presence of 33P-ATP by P81 ion exchange cellulose chromatographyMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibition of FLT3 D835Y mutant (unknown origin)More data for this Ligand-Target Pair

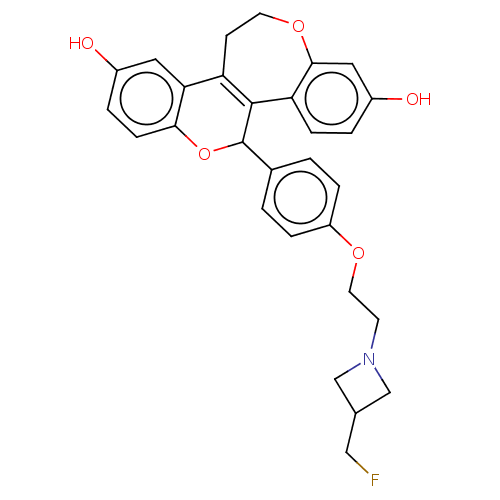
 3D Structure (crystal)
3D Structure (crystal)