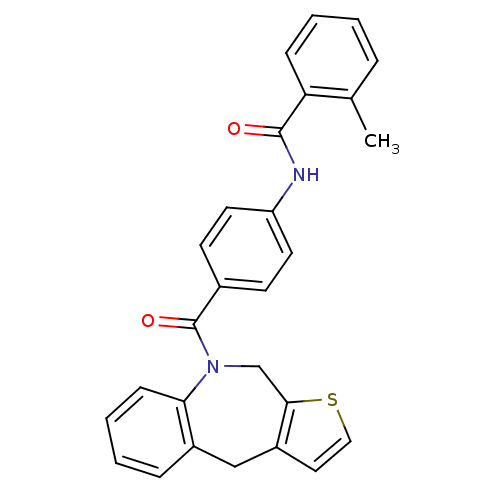
Affinity DataIC50: 0.700nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
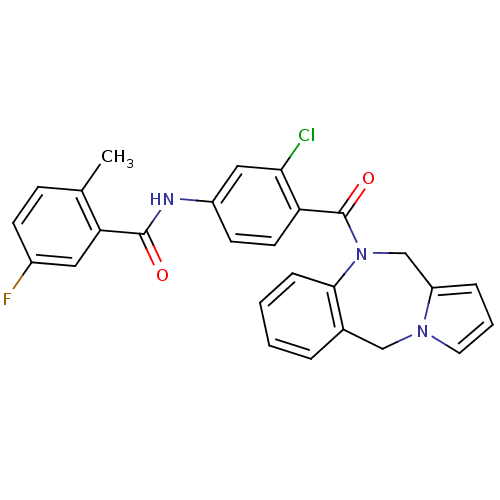
Affinity DataIC50: 1.20nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
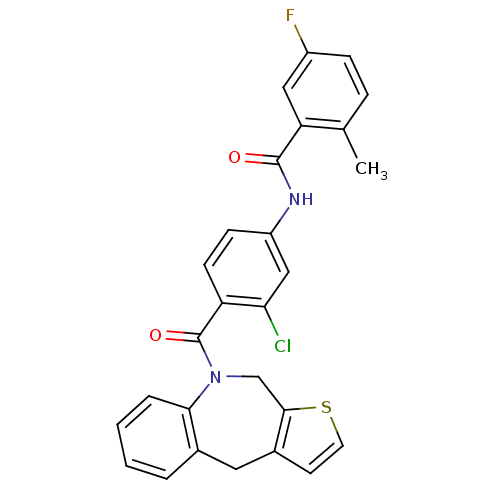
Affinity DataIC50: 6.80nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
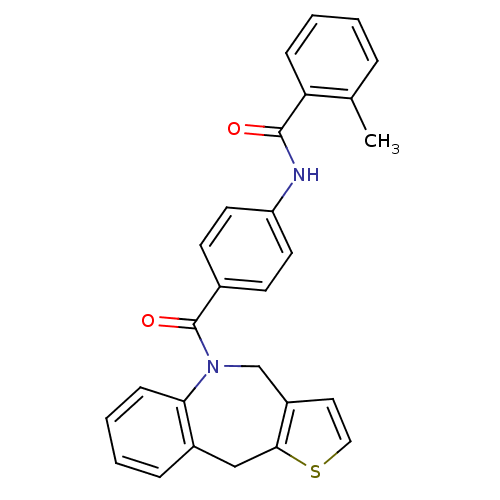
Affinity DataIC50: 8.5nMAssay Description:In vitro inhibition of [3H]- AVP binding to V1a receptor from human platelet membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2)More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:In vitro inhibition of [3H]-AVP binding to rat V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:In vitro inhibition of [3H]- AVP binding to V1a receptor from human platelet membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:In vitro inhibition of [3H]- AVP binding to V1a receptor from human platelet membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 59nMAssay Description:In vitro inhibition of [3H]- AVP binding to rat V1a receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:In vitro inhibition of [3H]- AVP binding to V1a receptor from human platelet membraneMore data for this Ligand-Target Pair