Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Trifunctional purine biosynthetic protein adenosine-3
Ligand
BDBM22585
Substrate
BDBM22589
Meas. Tech.
GAR Tfase Activity Assay
pH
7.5±n/a
Temperature
299.15±n/a K
Ki
>100000±n/a nM
Citation
 Xu, L; Chong, Y; Hwang, I; D'Onofrio, A; Amore, K; Beardsley, GP; Li, C; Olson, AJ; Boger, DL; Wilson, IA Structure-based design, synthesis, evaluation, and crystal structures of transition state analogue inhibitors of inosine monophosphate cyclohydrolase. J Biol Chem 282:13033-46 (2007) [PubMed] Article
Xu, L; Chong, Y; Hwang, I; D'Onofrio, A; Amore, K; Beardsley, GP; Li, C; Olson, AJ; Boger, DL; Wilson, IA Structure-based design, synthesis, evaluation, and crystal structures of transition state analogue inhibitors of inosine monophosphate cyclohydrolase. J Biol Chem 282:13033-46 (2007) [PubMed] Article More Info.:
Target
Name:
Trifunctional purine biosynthetic protein adenosine-3
Synonyms:
GAR Tfase | GAR transformylase | GART | Glycinamide ribonucleotide formyltransferase (GARFTase) | Glycinamide ribonucleotide transformylase (GAR Tfase) | PGFT | PRGS | PUR2_HUMAN | Thymidylate synthase/GAR transformylase/AICAR transformylase
Type:
Protein
Mol. Mass.:
107768.47
Organism:
Homo sapiens (Human)
Description:
P22102
Residue:
1010
Sequence:
MAARVLIIGSGGREHTLAWKLAQSHHVKQVLVAPGNAGTACSEKISNTAISISDHTALAQFCKEKKIEFVVVGPEAPLAAGIVGNLRSAGVQCFGPTAEAAQLESSKRFAKEFMDRHGIPTAQWKAFTKPEEACSFILSADFPALVVKASGLAAGKGVIVAKSKEEACKAVQEIMQEKAFGAAGETIVIEELLDGEEVSCLCFTDGKTVAPMPPAQDHKRLLEGDGGPNTGGMGAYCPAPQVSNDLLLKIKDTVLQRTVDGMQQEGTPYTGILYAGIMLTKNGPKVLEFNCRFGDPECQVILPLLKSDLYEVIQSTLDGLLCTSLPVWLENHTALTVVMASKGYPGDYTKGVEITGFPEAQALGLEVFHAGTALKNGKVVTHGGRVLAVTAIRENLISALEEAKKGLAAIKFEGAIYRKDVGFRAIAFLQQPRSLTYKESGVDIAAGNMLVKKIQPLAKATSRSGCKVDLGGFAGLFDLKAAGFKDPLLASGTDGVGTKLKIAQLCNKHDTIGQDLVAMCVNDILAQGAEPLFFLDYFSCGKLDLSVTEAVVAGIAKACGKAGCALLGGETAEMPDMYPPGEYDLAGFAVGAMERDQKLPHLERITEGDVVVGIASSGLHSNGFSLVRKIVAKSSLQYSSPAPDGCGDQTLGDLLLTPTRIYSHSLLPVLRSGHVKAFAHITGGGLLENIPRVLPEKLGVDLDAQTWRIPRVFSWLQQEGHLSEEEMARTFNCGVGAVLVVSKEQTEQILRDIQQHKEEAWVIGSVVARAEGSPRVKVKNLIESMQINGSVLKNGSLTNHFSFEKKKARVAVLISGTGSNLQALIDSTREPNSSAQIDIVISNKAAVAGLDKAERAGIPTRVINHKLYKNRVEFDSAIDLVLEEFSIDIVCLAGFMRILSGPFVQKWNGKMLNIHPSLLPSFKGSNAHEQALETGVTVTGCTVHFVAEDVDAGQIILQEAVPVKRGDTVATLSERVKLAEHKIFPAALQLVASGTVQLGENGKICWVKEE
Inhibitor
Name:
BDBM22585
Synonyms:
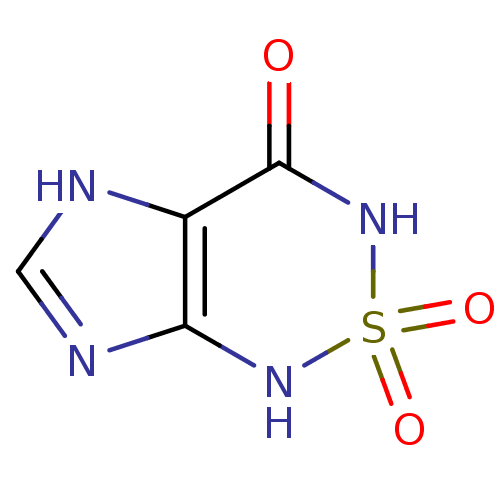
1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadiazine-2,2,4-trione | Heterocycle, 1
Type:
Small organic molecule
Emp. Form.:
C4H4N4O3S
Mol. Mass.:
188.165
SMILES:
O=C1NS(=O)(=O)Nc2nc[nH]c12
Substrate
Name:
BDBM22589
Synonyms:
GAR | Glycineamideribotide | glycineamide ribonucleotide | {[(2R,3S,4R,5R)-5-(2-aminoacetamido)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid
Type:
Nucleoside or nucleotide
Emp. Form.:
C7H15N2O8P
Mol. Mass.:
286.1764
SMILES:
NCC(=O)N[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O