Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
Ligand
BDBM25056
Substrate
BDBM15235
Meas. Tech.
Scintillation Proximity Assay
pH
7.4±n/a
Temperature
295.15±n/a K
IC50
100±n/a nM
Citation
 Hayakawa, M; Kaizawa, H; Kawaguchi, K; Ishikawa, N; Koizumi, T; Ohishi, T; Yamano, M; Okada, M; Ohta, M; Tsukamoto, S; Raynaud, FI; Waterfield, MD; Parker, P; Workman, P Synthesis and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem 15:403-12 (2007) [PubMed] Article
Hayakawa, M; Kaizawa, H; Kawaguchi, K; Ishikawa, N; Koizumi, T; Ohishi, T; Yamano, M; Okada, M; Ohta, M; Tsukamoto, S; Raynaud, FI; Waterfield, MD; Parker, P; Workman, P Synthesis and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem 15:403-12 (2007) [PubMed] Article More Info.:
Target
Name:
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
Synonyms:
C2-PI3K | P3C2B_HUMAN | PI3K-C2beta | PIK3C2B | Phosphatidylinositol 4-phosphate 3-kinase C2 beta (PIK3C2B) | Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing beta polypeptide | Phosphoinositide 3-Kinase (PI3K), C2beta | Phosphoinositide 3-Kinase-C2-beta | PtdIns-3-kinase C2 beta
Type:
Enzyme
Mol. Mass.:
184784.86
Organism:
Homo sapiens (Human)
Description:
O00750
Residue:
1634
Sequence:
MSSTQGNGEHWKSLESVGISRKELAMAEALQMEYDALSRLRHDKEENRAKQNADPSLISWDEPGVDFYSKPAGRRTDLKLLRGLSGSDPTLNYNSLSPQEGPPNHSTSQGPQPGSDPWPKGSLSGDYLYIFDGSDGGVSSSPGPGDIEGSCKKLSPPPLPPRASIWDTPPLPPRKGSPSSSKISQPSDINTFSLVEQLPGKLLEHRILEEEEVLGGGGQGRLLGSVDYDGINDAITRLNLKSTYDAEMLRDATRGWKEGRGPLDFSKDTSGKPVARSKTMPPQVPPRTYASRYGNRKNATPGKNRRISAAPVGSRPHTVANGHELFEVSEERDEEVAAFCHMLDILRSGSDIQDYFLTGYVWSAVTPSPEHLGDEVNLKVTVLCDRLQEALTFTCNCSSTVDLLIYQTLCYTHDDLRNVDVGDFVLKPCGLEEFLQNKHALGSHEYIQYCRKFDIDIRLQLMEQKVVRSDLARTVNDDQSPSTLNYLVHLQERPVKQTISRQALSLLFDTYHNEVDAFLLADGDFPLKADRVVQSVKAICNALAAVETPEITSALNQLPPCPSRMQPKIQKDPSVLAVRENREKVVEALTAAILDLVELYCNTFNADFQTAVPGSRKHDLVQEACHFARSLAFTVYATHRIPIIWATSYEDFYLSCSLSHGGKELCSPLQTRRAHFSKYLFHLIVWDQQICFPVQVNRLPRETLLCATLYALPIPPPGSSSEANKQRRVPEALGWVTTPLFNFRQVLTCGRKLLGLWPATQENPSARWSAPNFHQPDSVILQIDFPTSAFDIKFTSPPGDKFSPRYEFGSLREEDQRKLKDIMQKESLYWLTDADKKRLWEKRYYCHSEVSSLPLVLASAPSWEWACLPDIYVLLKQWTHMNHQDALGLLHATFPDQEVRRMAVQWIGSLSDAELLDYLPQLVQALKYECYLDSPLVRFLLKRAVSDLRVTHYFFWLLKDGLKDSQFSIRYQYLLAALLCCCGKGLREEFNRQCWLVNALAKLAQQVREAAPSARQGILRTGLEEVKQFFALNGSCRLPLSPSLLVKGIVPRDCSYFNSNAVPLKLSFQNVDPLGENIRVIFKCGDDLRQDMLTLQMIRIMSKIWVQEGLDMRMVIFRCFSTGRGRGMVEMIPNAETLRKIQVEHGVTGSFKDRPLADWLQKHNPGEDEYEKAVENFIYSCAGCCVATYVLGICDRHNDNIMLKTTGHMFHIDFGRFLGHAQMFGNIKRDRAPFVFTSDMAYVINGGDKPSSRFHDFVDLCCQAYNLIRKHTHLFLNLLGLMLSCGIPELSDLEDLKYVYDALRPQDTEANATTYFTRLIESSLGSVATKLNFFIHNLAQMKFTGSDDRLTLSFASRTHTLKSSGRISDVFLCRHEKIFHPNKGYIYVVKVMRENTHEATYIQRTFEEFQELHNKLRLLFPSSHLPSFPSRFVIGRSRGEAVAERRREELNGYIWHLIHAPPEVAECDLVYTFFHPLPRDEKAMGTSPAPKSSDGTWARPVGKVGGEVKLSISYKNNKLFIMVMHIRGLQLLQDGNDPDPYVKIYLLPDPQKTTKRKTKVARKTCNPTYNEMLVYDGIPKGDLQQRELQLSVLSEQGFWENVLLGEVNIRLRELDLAQEKTGWFALGSRSHGTL
Inhibitor
Name:
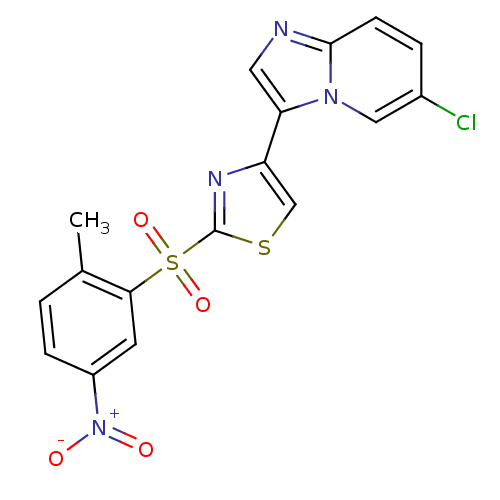
BDBM25056
Synonyms:
4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methyl-5-nitrobenzene)sulfonyl]-1,3-thiazole | imidazo[1,2-a]pyridine derivative, 12
Type:
Small organic molecule
Emp. Form.:
C17H11ClN4O4S2
Mol. Mass.:
434.877
SMILES:
Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O
Substrate
Name:
BDBM15235
Synonyms:
L-alpha-Phosphatidylinositol 4,5-bisphosphate | Phosphatidylinositol-4,5-bisphosphate (PIP2) | PtdInsP2
Type:
Small organic molecule
Emp. Form.:
C47H80O19P3
Mol. Mass.:
1042.0507
SMILES:
CCCCCCCCCCCCCCCCCC(=O)OCC(COP([O-])(=O)O[C@@H]1[C@H](O)[C@H](O)[C@@H](OP([O-])([O-])=O)[C@H](OP([O-])([O-])=O)[C@H]1O)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC