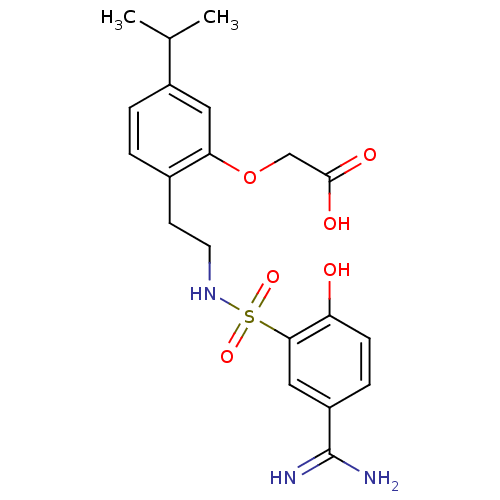
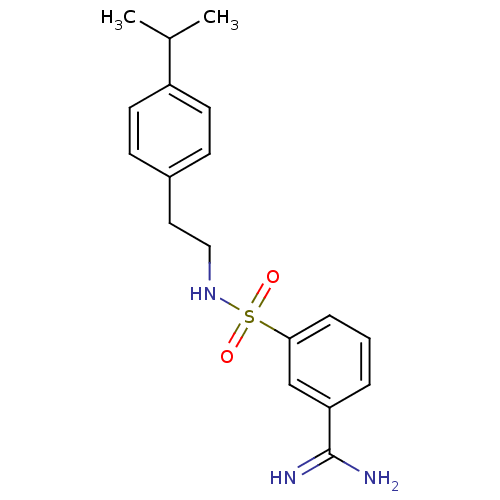
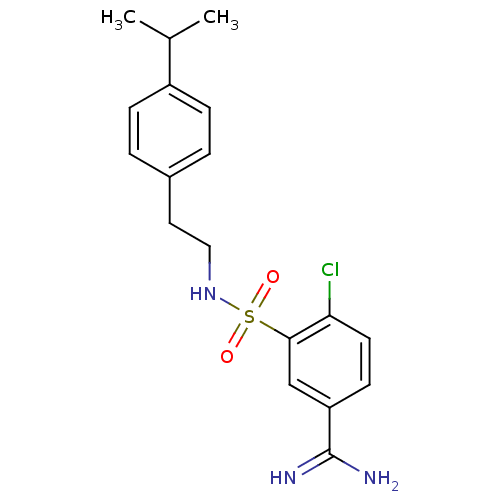
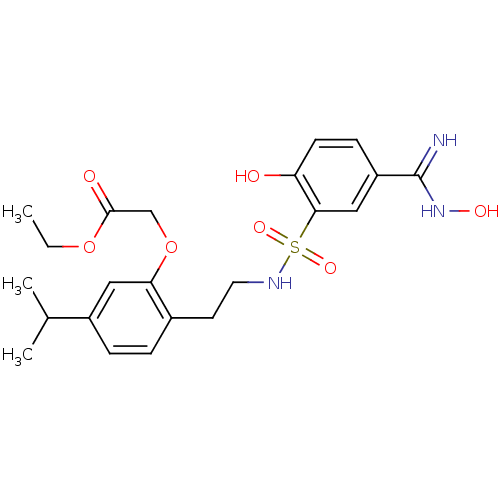
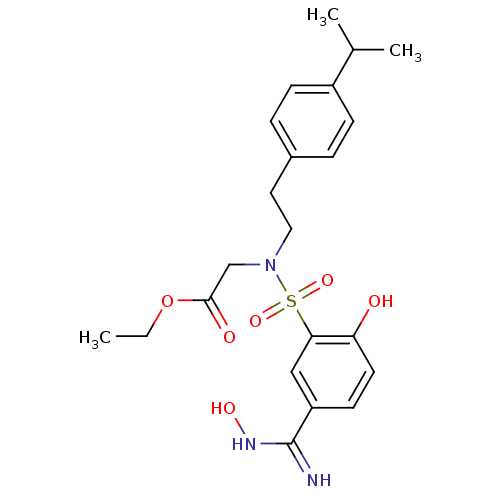
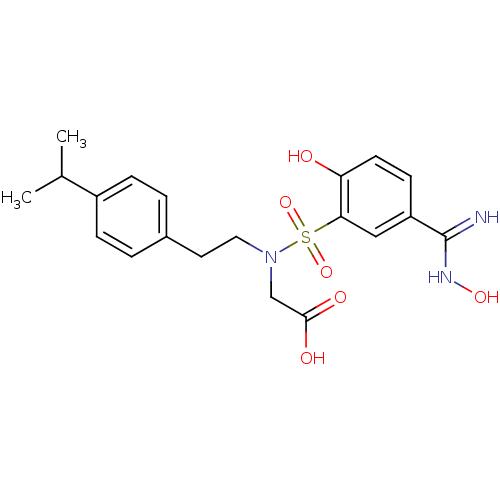
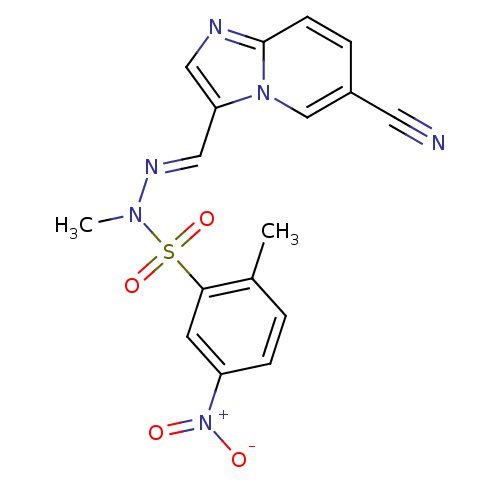
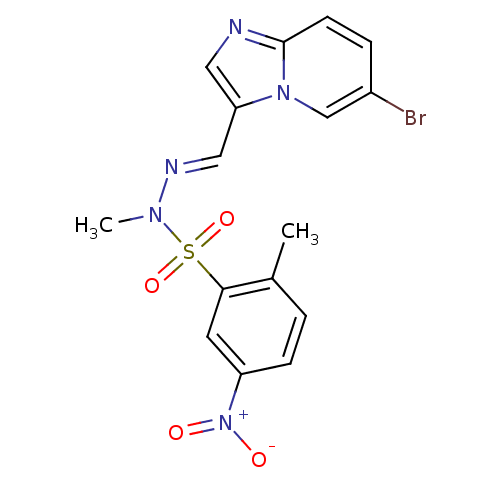
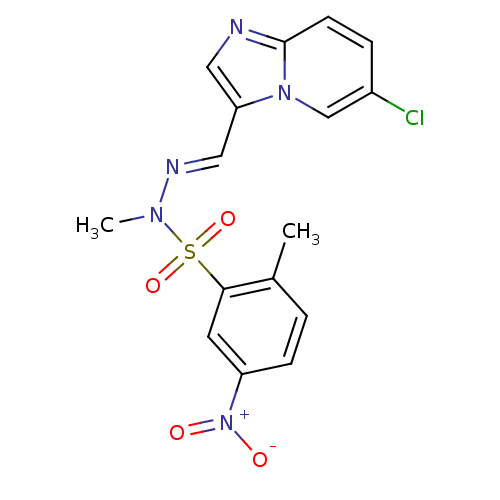
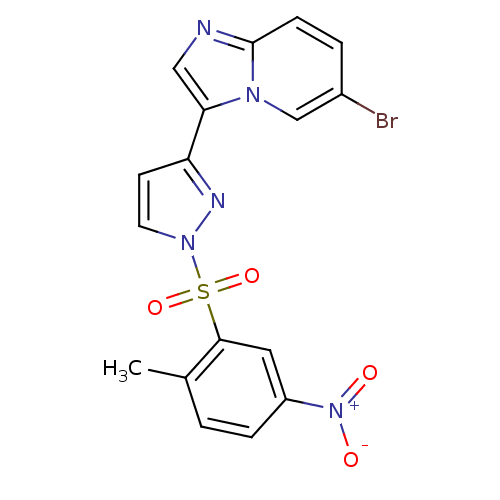
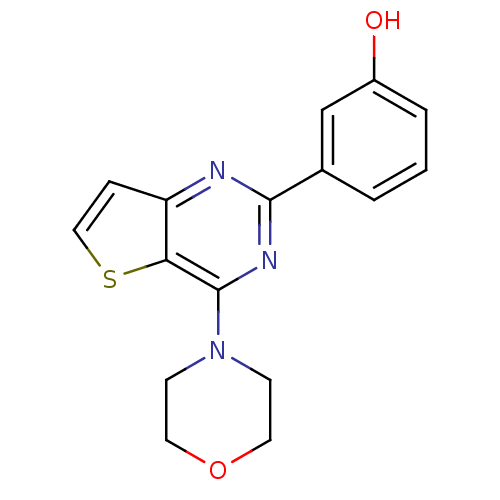
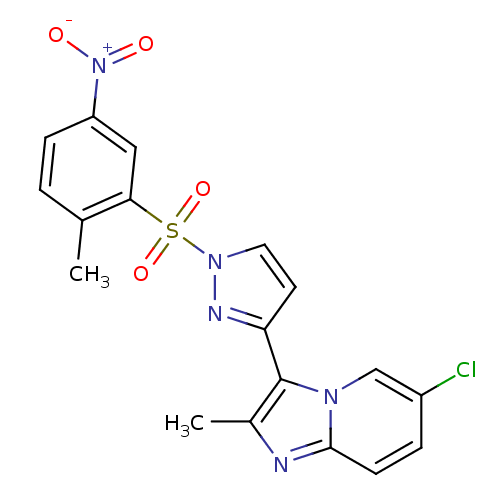
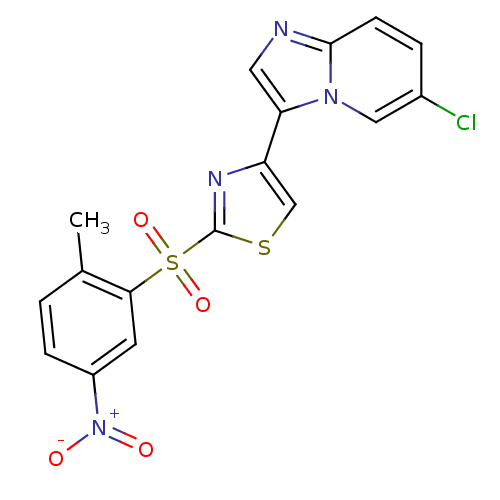
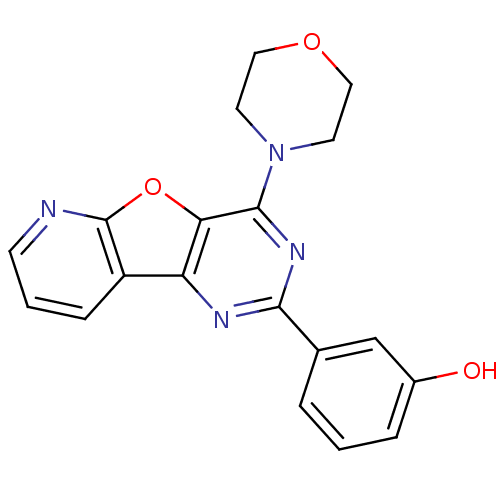
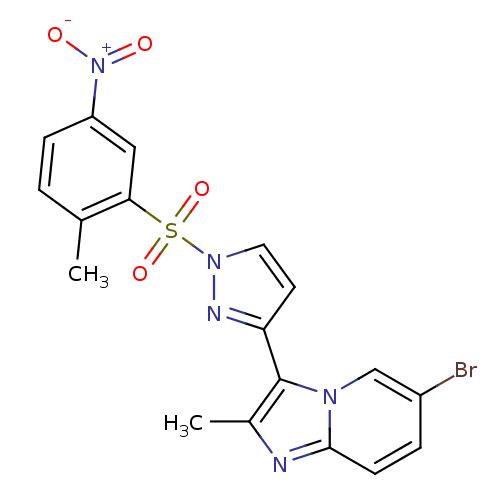
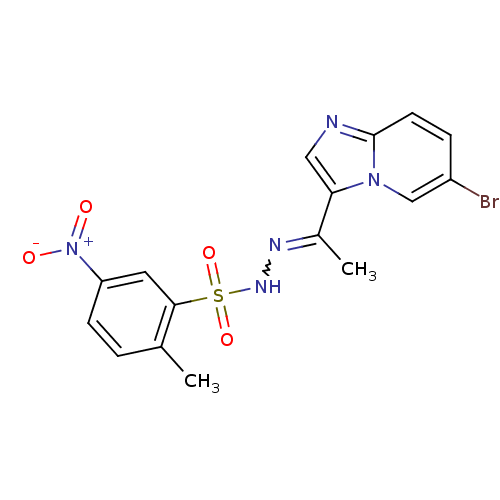
Affinity DataKi: 1.73nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
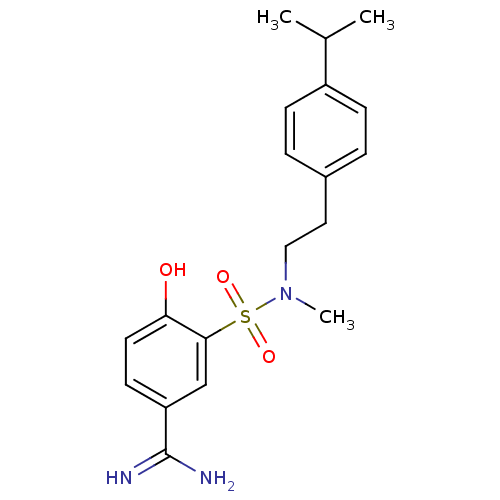
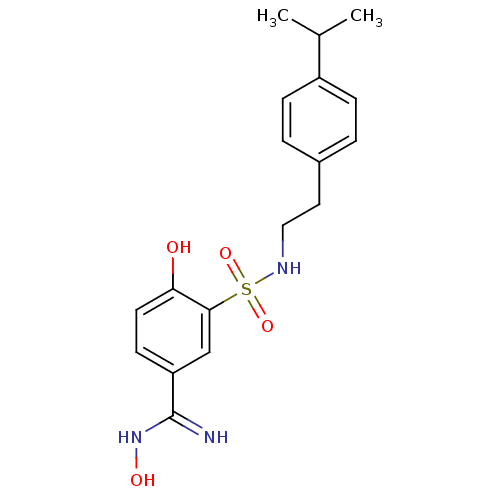
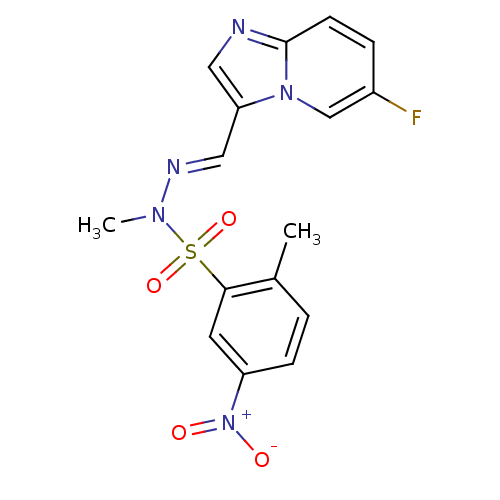
Affinity DataKi: 5nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
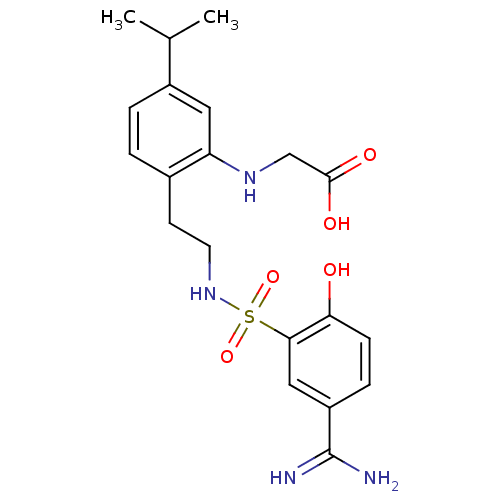
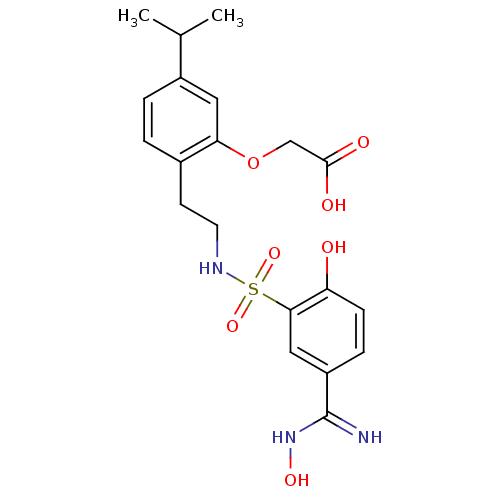
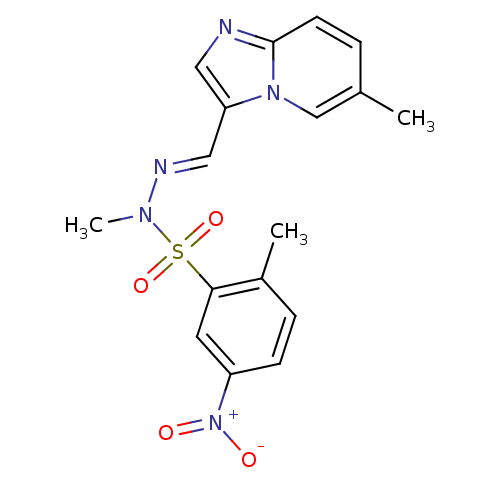
Affinity DataKi: 5.70nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
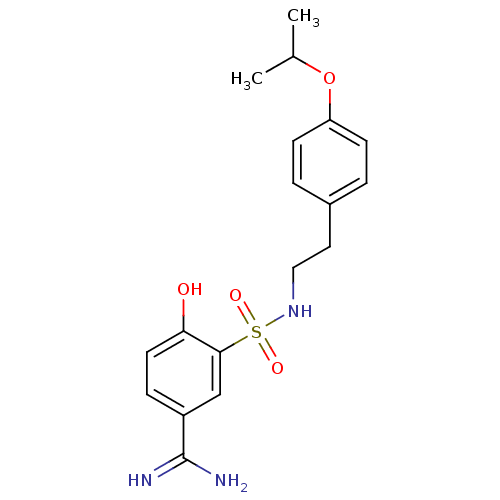
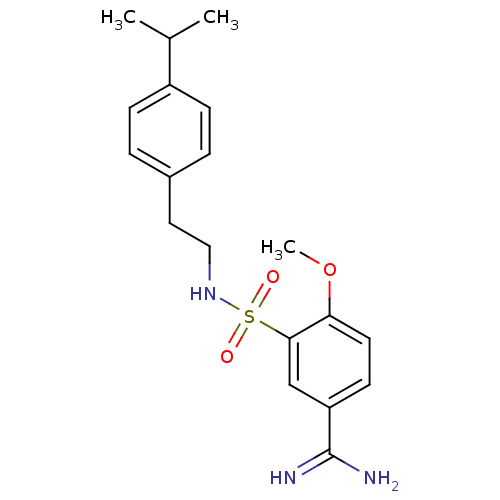
Affinity DataKi: 9.70nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Inhibition of factor 10a in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+3nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.30E+3nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of human thrombin by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of human thrombin by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of human thrombin by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of human factor 10a by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+4nMAssay Description:Inhibition of human thrombin by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: 5.78E+4nMAssay Description:Inhibition of human thrombin by Dixon-plot methodMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+6nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
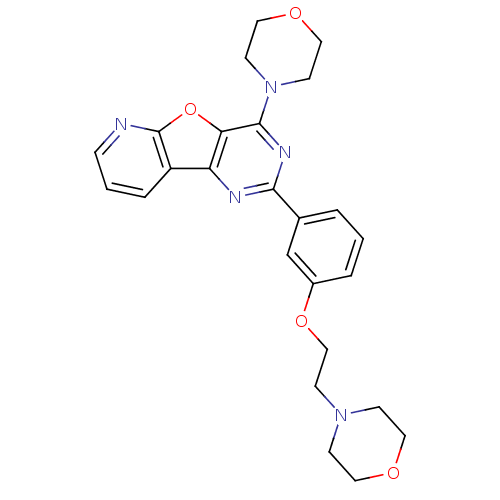
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 0.260nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 0.770nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 1.80nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 2.5nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 2.5nMAssay Description:Inhibition of p110alpha by SPA assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 2.80nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 2.80nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 3nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 3.10nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 3.10nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 3.60nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 3.60nMAssay Description:Inhibition of p110alpha by SPA assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 5.30nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 6nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta(Homo sapiens (Human))
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of PI3Kc2betaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta(Homo sapiens (Human))
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 10nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 14nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 16nMAssay Description:Inhibition of p110alpha by SPA assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 16nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astellas Pharma
Curated by ChEMBL
Astellas Pharma
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of p110betaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astellas Pharma
Astellas Pharma
Affinity DataIC50: 17nMpH: 7.4 T: 2°CAssay Description:The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft...More data for this Ligand-Target Pair

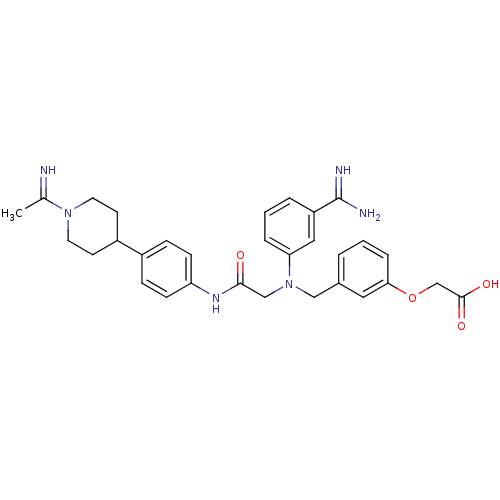
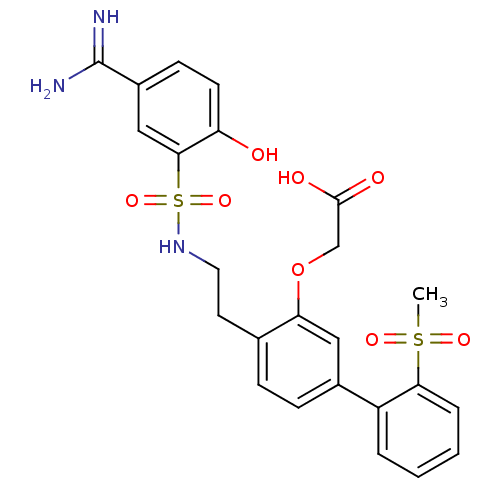
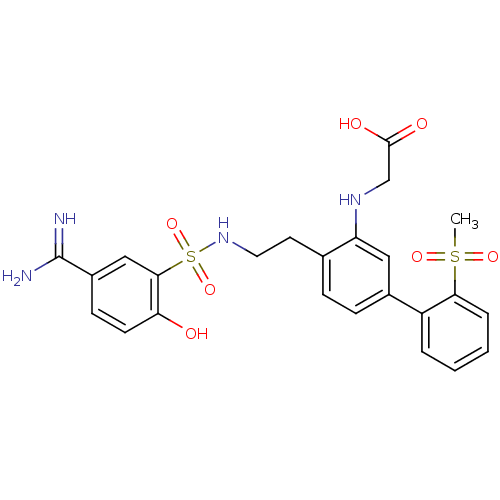
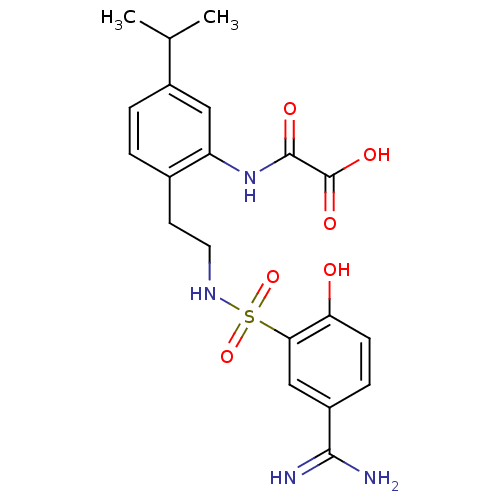
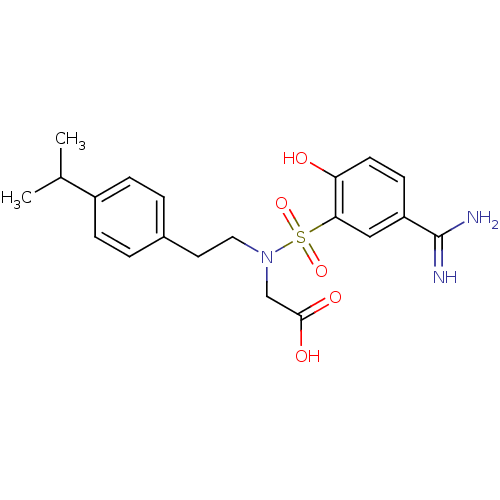
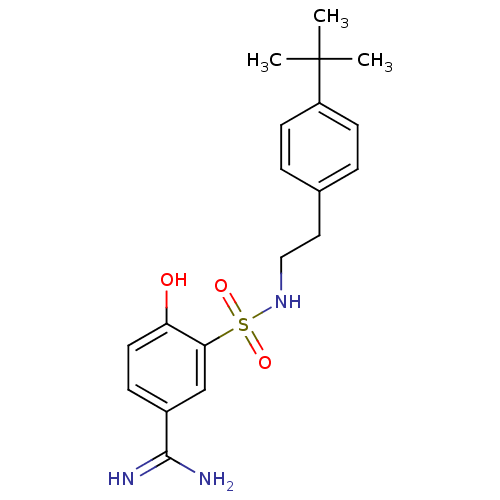
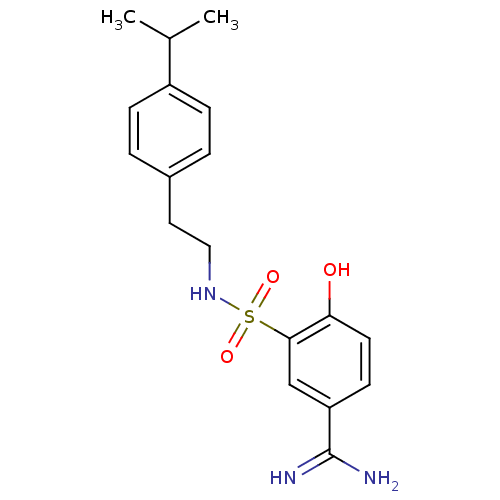
 3D Structure (crystal)
3D Structure (crystal)