Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2A
Ligand
BDBM21398
Substrate
n/a
Meas. Tech.
ChEMBL_449893 (CHEMBL898999)
Ki
35±n/a nM
Citation
 Chakrabarty, R; Rao, J; Anand, A; Roy, AD; Roy, R; Shankar, G; Dua, PR; Saxena, AK Rational design, synthesis and evaluation of (6aR( *),11bS( *))-1-(4-fluorophenyl)-4-{7-[4-(4-fluorophenyl)-4-oxobutyl]1,2,3,4,6,6a,7,11b,12,12a(RS)-decahydropyrazino[2',1':6,1]pyrido[3,4-b]indol-2-yl}-butan-1-one as a potential neuroleptic agent. Bioorg Med Chem 15:7361-7 (2007) [PubMed] Article
Chakrabarty, R; Rao, J; Anand, A; Roy, AD; Roy, R; Shankar, G; Dua, PR; Saxena, AK Rational design, synthesis and evaluation of (6aR( *),11bS( *))-1-(4-fluorophenyl)-4-{7-[4-(4-fluorophenyl)-4-oxobutyl]1,2,3,4,6,6a,7,11b,12,12a(RS)-decahydropyrazino[2',1':6,1]pyrido[3,4-b]indol-2-yl}-butan-1-one as a potential neuroleptic agent. Bioorg Med Chem 15:7361-7 (2007) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2A | 5-HT2 | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_RAT | Htr2 | Htr2a | Serotonin Receptor 2A
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
52852.05
Organism:
Rattus norvegicus (rat)
Description:
Rat cortex membranes 5-HT2A receptors.
Residue:
471
Sequence:
MEILCEDNISLSSIPNSLMQLGDGPRLYHNDFNSRDANTSEASNWTIDAENRTNLSCEGYLPPTCLSILHLQEKNWSALLTTVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAIWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVAFFIPLTIMVITYFLTIKSLQKEATLCVSDLSTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYAGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNENVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENRKPLQLILVNTIPALAYKSSQLQVGQKKNSQEDAEQTVDDCSMVTLGKQQSEENCTDNIETVNEKVSCV
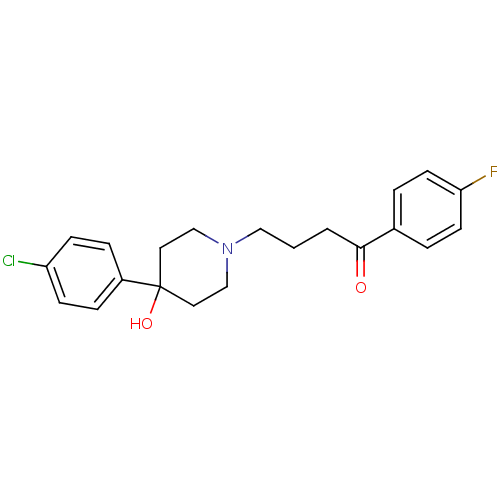
Inhibitor
Name:
BDBM21398
Synonyms:
4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-butan-1-one;propionate(HCl) | 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one | CHEMBL54 | CHEMBL545608 | Haloperidol | Haloperidol, 1
Type:
Small organic molecule
Emp. Form.:
C21H23ClFNO2
Mol. Mass.:
375.864
SMILES:
OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1