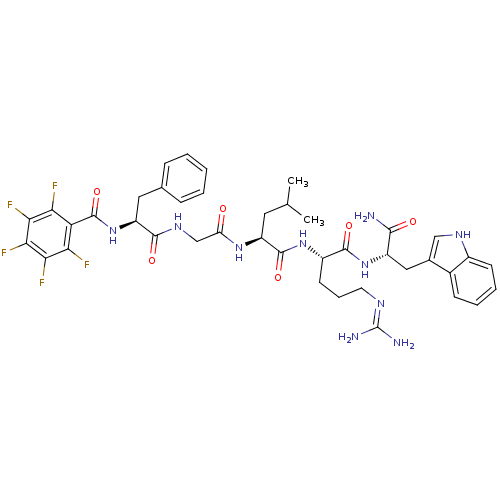
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
KiSS-1 receptor
Ligand
BDBM50216086
Substrate
n/a
Meas. Tech.
ChEMBL_439840 (CHEMBL890161)
EC50
>100±n/a nM
Citation
 Tomita, K; Oishi, S; Cluzeau, J; Ohno, H; Navenot, JM; Wang, ZX; Peiper, SC; Akamatsu, M; Fujii, N SAR and QSAR studies on the N-terminally acylated pentapeptide agonists for GPR54. J Med Chem 50:3222-8 (2007) [PubMed] Article
Tomita, K; Oishi, S; Cluzeau, J; Ohno, H; Navenot, JM; Wang, ZX; Peiper, SC; Akamatsu, M; Fujii, N SAR and QSAR studies on the N-terminally acylated pentapeptide agonists for GPR54. J Med Chem 50:3222-8 (2007) [PubMed] Article More Info.:
Target
Name:
KiSS-1 receptor
Synonyms:
AXOR12 | G-protein Coupled Receptor 54 | G-protein coupled receptor 54 (GPR54) | GPR54 | Hypogonadotropin-1 | KISS1R | KISSR_HUMAN | KiSS-1R | Kisspeptins receptor | Metastin receptor | hOT7T175
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
42613.79
Organism:
Homo sapiens (Human)
Description:
Binding assay was performed using membranes from the CHO cell transfectants.
Residue:
398
Sequence:
MHTVATSGPNASWGAPANASGCPGCGANASDGPVPSPRAVDAWLVPLFFAALMLLGLVGNSLVIYVICRHKPMRTVTNFYIANLAATDVTFLLCCVPFTALLYPLPGWVLGDFMCKFVNYIQQVSVQATCATLTAMSVDRWYVTVFPLRALHRRTPRLALAVSLSIWVGSAAVSAPVLALHRLSPGPRAYCSEAFPSRALERAFALYNLLALYLLPLLATCACYAAMLRHLGRVAVRPAPADSALQGQVLAERAGAVRAKVSRLVAAVVLLFAACWGPIQLFLVLQALGPAGSWHPRSYAAYALKTWAHCMSYSNSALNPLLYAFLGSHFRQAFRRVCPCAPRRPRRPRRPGPSDPAAPHAELLRLGSHPAPARAQKPGSSGLAARGLCVLGEDNAPL
Inhibitor
Name:
BDBM50216086
Synonyms:
(2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoyl-2-(1H-indol-3-yl)ethyl]carbamoyl}butyl]-4-methyl-2-{2-[(2S)-2-[(2,3,4,5,6-pentafluorophenyl)formamido]-3-phenylpropanamido]acetamido}pentanamide | CHEMBL415221
Type:
Small organic molecule
Emp. Form.:
C41H47F5N10O6
Mol. Mass.:
870.8673
SMILES:
CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1c(F)c(F)c(F)c(F)c1F)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |wU:12.20,4.4,48.49,wD:37.38,(11.42,-13.89,;11.42,-15.43,;12.75,-16.21,;10.08,-16.21,;10.08,-17.75,;8.75,-18.51,;7.42,-17.74,;7.42,-16.19,;6.08,-18.51,;4.74,-17.75,;3.41,-18.51,;3.41,-20.06,;2.08,-17.75,;2.08,-16.21,;3.41,-15.43,;4.74,-16.2,;6.08,-15.43,;6.07,-13.89,;4.74,-13.12,;3.4,-13.89,;.74,-18.52,;-.59,-17.74,;-.59,-16.21,;-1.91,-18.51,;-1.91,-20.04,;-.58,-20.8,;-3.23,-20.81,;-3.23,-22.34,;-4.57,-20.04,;-5.9,-20.81,;-4.57,-18.5,;-5.9,-17.73,;-3.24,-17.74,;-3.24,-16.2,;11.42,-18.51,;11.42,-20.05,;12.75,-17.74,;14.09,-18.5,;14.09,-20.04,;15.42,-20.81,;15.42,-22.35,;16.76,-23.12,;16.76,-24.66,;15.43,-25.43,;18.09,-25.44,;15.42,-17.72,;15.42,-16.18,;16.75,-18.5,;18.09,-17.73,;18.09,-16.18,;19.42,-15.42,;20.82,-16.04,;21.85,-14.9,;21.08,-13.56,;21.56,-12.09,;20.52,-10.95,;19.01,-11.27,;18.54,-12.74,;19.58,-13.88,;19.42,-18.5,;20.75,-17.73,;19.42,-20.04,)|