Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 1
Ligand
BDBM17638
Substrate
n/a
Meas. Tech.
ChEMBL_849361 (CHEMBL2149153)
IC50
2100±n/a nM
Citation
 Baumgartner, L; Sosa, S; Atanasov, AG; Bodensieck, A; Fakhrudin, N; Bauer, J; Favero, GD; Ponti, C; Heiss, EH; Schwaiger, S; Ladurner, A; Widowitz, U; Loggia, RD; Rollinger, JM; Werz, O; Bauer, R; Dirsch, VM; Tubaro, A; Stuppner, H Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. J Nat Prod 74:1779-86 (2011) [PubMed] Article
Baumgartner, L; Sosa, S; Atanasov, AG; Bodensieck, A; Fakhrudin, N; Bauer, J; Favero, GD; Ponti, C; Heiss, EH; Schwaiger, S; Ladurner, A; Widowitz, U; Loggia, RD; Rollinger, JM; Werz, O; Bauer, R; Dirsch, VM; Tubaro, A; Stuppner, H Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. J Nat Prod 74:1779-86 (2011) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 1
Synonyms:
COX1 | Cyclooxygenase-1 | Cyclooxygenase-1 (COX-1) | PGH1_SHEEP | PTGS1 | Prostaglandin G/H synthase (cyclooxygenase)
Type:
Protein
Mol. Mass.:
68868.60
Organism:
Ovis aries (Sheep)
Description:
n/a
Residue:
600
Sequence:
MSRQSISLRFPLLLLLLSPSPVFSADPGAPAPVNPCCYYPCQHQGICVRFGLDRYQCDCTRTGYSGPNCTIPEIWTWLRTTLRPSPSFIHFLLTHGRWLWDFVNATFIRDTLMRLVLTVRSNLIPSPPTYNIAHDYISWESFSNVSYYTRILPSVPRDCPTPMDTKGKKQLPDAEFLSRRFLLRRKFIPDPQSTNLMFAFFAQHFTHQFFKTSGKMGPGFTKALGHGVDLGHIYGDNLERQYQLRLFKDGKLKYQMLNGEVYPPSVEEAPVLMHYPRGIPPQSQMAVGQEVFGLLPGLMLYATIWLREHNRVCDLLKAEHPTWGDEQLFQTARLILIGETIKIVIEEYVQQLSGYFLQLKFDPELLFGAQFQYRNRIAMEFNQLYHWHPLMPDSFRVGPQDYSYEQFLFNTSMLVDYGVEALVDAFSRQPAGRIGGGRNIDHHILHVAVDVIKESRVLRLQPFNEYRKRFGMKPYTSFQELTGEKEMAAELEELYGDIDALEFYPGLLLEKCHPNSIFGESMIEMGAPFSLKGLLGNPICSPEYWKASTFGGEVGFNLVKTATLKKLVCLNTKTCPYVSFHVPDPRQEDRPGVERPPTEL
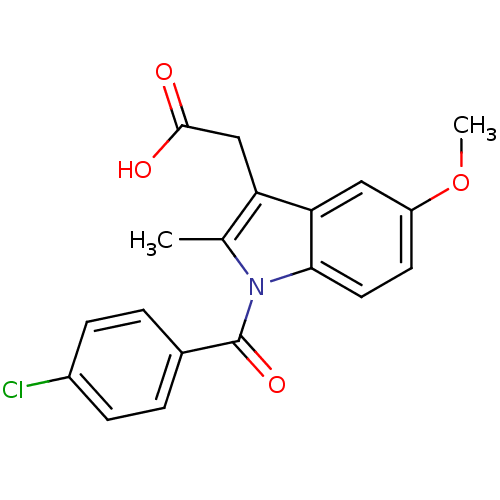
Inhibitor
Name:
BDBM17638
Synonyms:
2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetic acid | CHEMBL6 | Indocin | Indomethacin | US11478464, Compound Indomethacin | US11786535, Compound Indomethacin | US9271961, Indomethacin | indometacin
Type:
Small organic molecule
Emp. Form.:
C19H16ClNO4
Mol. Mass.:
357.788
SMILES:
COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1