Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416]
Ligand
BDBM244792
Substrate
n/a
Meas. Tech.
In Vitro Biological Assay
IC50
0.59±n/a nM
Citation
 Klaus, C; Raimondi, MA; Daigle, SR; Pollock, RM Combination therapy for treating cancer US Patent US9446064 Publication Date 9/20/2016
Klaus, C; Raimondi, MA; Daigle, SR; Pollock, RM Combination therapy for treating cancer US Patent US9446064 Publication Date 9/20/2016 More Info.:
Target
Name:
Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416]
Synonyms:
DOT1L | DOT1L_HUMAN | Histone H3-K79 methyltransferase (DOT1L) | KIAA1814 | KMT4
Type:
Protein
Mol. Mass.:
47443.16
Organism:
Homo sapiens (Human)
Description:
Q8TEK3 aa 1-416
Residue:
416
Sequence:
MGEKLELRLKSPVGAEPAVYPWPLPVYDKHHDAAHEIIETIRWVCEEIPDLKLAMENYVLIDYDTKSFESMQRLCDKYNRAIDSIHQLWKGTTQPMKLNTRPSTGLLRHILQQVYNHSVTDPEKLNNYEPFSPEVYGETSFDLVAQMIDEIKMTDDDLFVDLGSGVGQVVLQVAAATNCKHHYGVEKADIPAKYAETMDREFRKWMKWYGKKHAEYTLERGDFLSEEWRERIANTSVIFVNNFAFGPEVDHQLKERFANMKEGGRIVSSKPFAPLNFRINSRNLSDIGTIMRVVELSPLKGSVSWTGKPVSYYLHTIDRTILENYFSSLKNPKLREEQEAARRRQQRESKSNAATPTKGPEGKVAGPADAPMDSGAEEEKAGAATVKKPSPSKARKKKLNKKGRKMAGRKRGRPKK
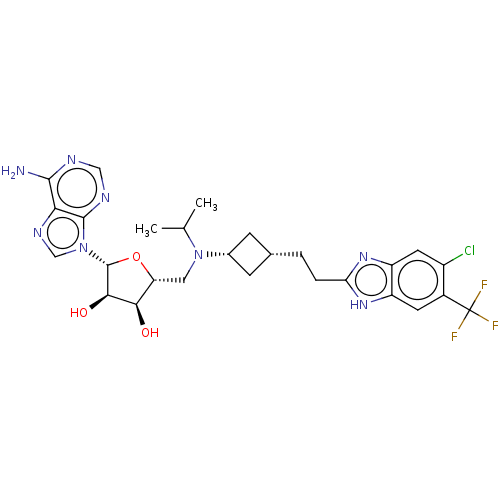
Inhibitor
Name:
BDBM244792
Synonyms:
US9446064, A5
Type:
Small organic molecule
Emp. Form.:
C27H32ClF3N8O3
Mol. Mass.:
609.043
SMILES:
CC(C)N(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@H](CCc2nc3cc(Cl)c(cc3[nH]2)C(F)(F)F)C1 |r,wU:24.27,22.24,5.4,7.12,wD:10.11,8.8,(-3.22,.18,;-1.89,.95,;-1.89,2.49,;-.55,.18,;.78,.95,;2.11,.18,;3.36,1.09,;4.61,.18,;4.13,-1.28,;4.9,-2.61,;2.59,-1.28,;1.82,-2.61,;5.38,1.52,;4.47,2.76,;5.38,4.01,;6.84,3.53,;8.17,4.3,;8.17,5.84,;9.51,3.53,;9.51,1.99,;8.17,1.22,;6.84,1.99,;-.55,-1.36,;-1.64,-2.44,;-.55,-3.53,;-.55,-5.07,;-1.89,-5.84,;-3.22,-5.07,;-3.24,-3.61,;-4.89,-3.22,;-5.66,-1.89,;-7.2,-1.89,;-7.97,-.55,;-7.97,-3.22,;-7.2,-4.56,;-5.66,-4.56,;-4.63,-5.7,;-9.51,-3.22,;-10.28,-1.89,;-10.28,-4.56,;-8.74,-4.56,;.54,-2.44,)|