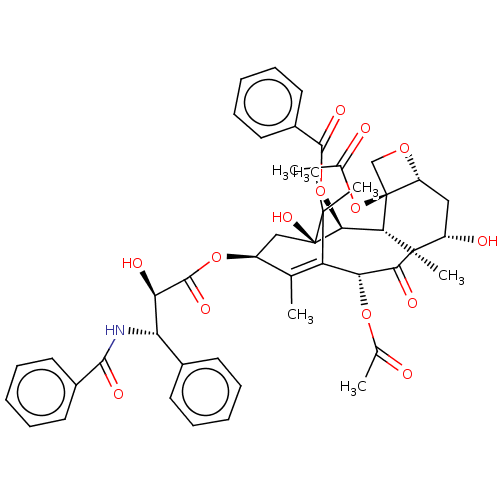
BDBM50001839 CHEMBL428647::PACLITAXEL::taxol
SMILES CC1=C2[C@H](C(=O)[C@@]3([C@H](C[C@@H]4[C@]([C@H]3[C@@H]([C@@](C2(C)C)(C[C@@H]1OC(=O)[C@@H]([C@H](c5ccccc5)NC(=O)c6ccccc6)O)O)OC(=O)c7ccccc7)(CO4)OC(=O)C)O)C)OC(=O)C
InChI Key InChIKey=RCINICONZNJXQF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 42 hits for monomerid = 50001839
Found 42 hits for monomerid = 50001839
Affinity DataIC50: 7.70nMAssay Description:Inhibition of beta3 tubulin overexpressed in human HeLa cells after 48 hrs by SRB assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of beta3 Tubulin (unknown origin) expressed in human HeLa cell line assessed as inhibition of cell proliferation measured after 48 hrs by ...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of human metastatic breast cancer (MDA-MB-435) cell proliferationMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Human)
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of human OATP1B3-mediated [3H]CCK-8 after 5 mins by Dixon plot methodMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Human)
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of human OATP1B1-mediated [3H]estrone 3-sulfate at after 5 mins by Dixon plot methodMore data for this Ligand-Target Pair
Affinity DataEC50: 520nMAssay Description:Induction of porcine brain tubulin polymerization after 20 mins by Bradford assayMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Human)
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Affinity DataKi: 840nMAssay Description:Inhibition of human OATP1B1-mediated [3H]estrone 3-sulfate at after 5 mins by Dixon plot methodMore data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus genotype 1b (isolate Con1) (HCV))
University of Wollongong
Curated by ChEMBL
University of Wollongong
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against hepatitis C virus helicaseMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Human)
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Universit£Tsmedizin G£Ttingen
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human OATP1B3-mediated [3H]CCK-8 after 5 mins by Dixon plot methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of MDR1 (unknown origin) transfected in human SKOV3 cells assessed as growth inhibition after 48 hrs by SRB assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of p-gp overexpressed in human SKOV3/M6-6 isogenic cells after 48 hrs by sulforhodamine B assayMore data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A4(Human)
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 4.90E+3nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Human)
Beijing Institute of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
Lakehead University and Thunder Bay Regional Research Institute
Curated by ChEMBL
Lakehead University and Thunder Bay Regional Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of Clostridium botulinum recombinant neurotoxin A light chain using SNAPtide as substrate after 1 hr by FRET assayMore data for this Ligand-Target Pair
TargetNucleotide-binding oligomerization domain-containing protein 2(Human)
Tsinghua University
Curated by ChEMBL
Tsinghua University
Curated by ChEMBL
Affinity DataIC50: 7.94E+3nMAssay Description:Inhibition of MDP-induced human NOD2 signaling expressed in HEK-Blue cells preincubated for 3 hrs followed by MDP-stimulation for 20 hrs by spectroph...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Src (unknown origin) assessed as incorporation of [32P] gamma-ATP in to myelin basic protein after 30 mins by autoradiographic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of EGFR (unknown origin) assessed as incorporation of [32P] gamma-ATP in to myelin basic protein after 30 mins by autoradiographic analysi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of FGFR1 (unknown origin) assessed as incorporation of [32P] gamma-ATP in to myelin basic protein after 30 mins by autoradiographic analys...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Abl1 (unknown origin) assessed as incorporation of [32P] gamma-ATP in to myelin basic protein after 30 mins by autoradiographic analysi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of recombinant human BSEP expressed in baculovirus infected sf9 cell plasma membrane vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataKi: 1.65E+4nM IC50: 4.20E+4nMpH: 10.5Assay Description:Paraoxonase enzyme activity was determined at 25 °C with paraoxon (1 mM) in 50 mM glycine-NaOH (pH 10.5) containing 1 mM CaCl2. The enzyme assay was ...More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Human)
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 2.00E+4nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
Affinity DataIC50: 2.44E+4nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.68E+4nMAssay Description:TP_TRANSPORTER: increase in bodipy intracellular accumulation (Bodipy: 0.2 uM) in SK-E2 cells (expressing BSEP)More data for this Ligand-Target Pair
Affinity DataIC50: 2.89E+4nMAssay Description:TP_TRANSPORTER: increase in dihydrofluorescein intracellular accumulation (dihydrofluorescein: 1 uM) in SK-E2 cells (expressing BSEP)More data for this Ligand-Target Pair
Affinity DataIC50: 5.39E+4nMAssay Description:TP_TRANSPORTER: inhibition of LDS-751 efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+4nMAssay Description:TP_TRANSPORTER: inhibition of Daunorubicin efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+4nMAssay Description:Inhibition of Escherichia coli GroEL expressed in Escherichia coliDH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed a...More data for this Ligand-Target Pair
Affinity DataIC50: 7.02E+4nMAssay Description:TP_TRANSPORTER: inhibition of Rhodamine 123 efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1-6(Human)
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 7.40E+4nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ...More data for this Ligand-Target Pair
Target60 kDa heat shock protein, mitochondrial(Human)
Indiana University School of Medicine
Curated by ChEMBL
Indiana University School of Medicine
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human N-terminal octa-His-tagged HSP60 expressed in Escherichia coli Rosetta(DE3) pLysS/human HSP10 expressed in Escherichia coli Roset...More data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+5nMAssay Description:Inhibition of Escherichia coli GroEL expressed in Escherichia coli DH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus type 1)
University of Illinois
Curated by ChEMBL
University of Illinois
Curated by ChEMBL
Affinity DataIC50: 2.34E+5nMAssay Description:Inhibition of HIV1 RTMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of ATPase activity of Escherichia coli GroEL expressed in Escherichia coliDH5alpha incubated for 60 mins using ATP by spectrometric analys...More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 2B7(Human)
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 3.00E+5nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 2B10(Human)
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 3.00E+5nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+5nMT: 2°CAssay Description:For the data shown, 25 nM BD-verapamil was added to various P-gp nanodisc concentrations in the presence of 1 μM empty nanodiscs, and 50 nM BD-v...More data for this Ligand-Target Pair