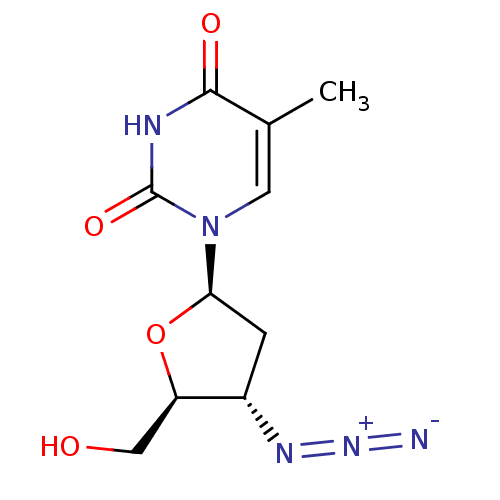
BDBM50002692 (AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione::(AZT)1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione::1-((2R,4R,5S)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione::1-((2R,4S,5S)-4-(diazoamino)-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione::1-((2R,4S,5S)-4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione::1-((2R,4S,5S)-4-azido-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione::1-((2R,4S,5S)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione::1-((2R,5S)-4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione::1-((2S,4R,5R)-4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione (AZT)::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione (AzddThd, AZT)::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione (N3ddThd)::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione [AZT]::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(3'-azido-2',3'-dideoxythymidine)::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(AZT)::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(Zidovudine, AZT)::1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(azidothymidine, AZT)::3'-Azido-3'-deoxy-thymidine::3'-Deoxy-3-azidothymidine::3'-azido-2',3'-dideoxythymidine::3'-azido-thymidine::3'azido-2'3'-dideoxythymidine::3-((2S,3S,5R)-2-(hydroxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3-yl)triaz-1-en-2-ium-1-ide::4-(4-Azido-5-hydroxy-tetrahydro-furan-2-yl)-5-methyl-3H-pyrazine-2,6-dione::AZT::BW-A-509U::BWA509U::CHEMBL129::Retrovir::US10071110, Compound AZT::US11420959, Example AZT::ZIDOVUDINE::azidothymidine::zidovudin::zudovidine
SMILES CC1=CN(C(=O)NC1=O)[C@H]2C[C@@H]([C@H](O2)CO)N=[N+]=[N-]
InChI Key InChIKey=HBOMLICNUCNMMY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 69 hits for monomerid = 50002692
Found 69 hits for monomerid = 50002692
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Nci-Frederick
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
University of Minnesota
Curated by ChEMBL
Institut Curie
Curated by ChEMBL
Ghent University
Curated by ChEMBL
Ghent University
Curated by ChEMBL