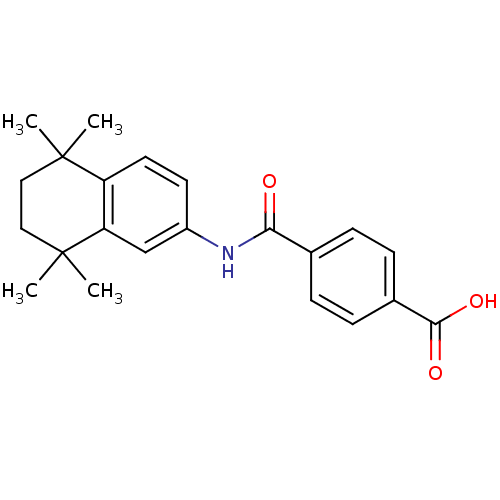
BDBM50061625 4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbamoyl]benzoic acid::Am 80::CHEMBL25202::Tamibarotene::US11744839, Compound of formula 3::US9963439, AM80::retinobenzoic acid
SMILES CC1(CCC(c2c1ccc(c2)NC(=O)c3ccc(cc3)C(=O)O)(C)C)C
InChI Key InChIKey=MUTNCGKQJGXKEM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50061625
Found 14 hits for monomerid = 50061625
Affinity DataKi: 6.5nMAssay Description:Agonistic activity towards retinoic acid receptor-alphaMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Agonistic activity towards retinoic acid receptor-betaMore data for this Ligand-Target Pair
Affinity DataEC50: 45nMAssay Description:Agonist activity at RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataEC50: 235nMAssay Description:Agonist activity at RARbetaMore data for this Ligand-Target Pair
Affinity DataEC50: 591nMAssay Description:Agonist activity at RARgammaMore data for this Ligand-Target Pair
Affinity DataKd: 1.38E+3nMAssay Description:Binding affinity to human N-terminal His6-tagged and thrombin cleavage fused BLVRB expressed in Escherichia coli BL21 (DE3) assessed as dissociation ...More data for this Ligand-Target Pair
Affinity DataKd: 1.38E+3nMAssay Description:Binding affinity to human N-terminal His6-tagged and thrombin cleavage fused BLVRB expressed in Escherichia coli BL21 (DE3) assessed as dissociation ...More data for this Ligand-Target Pair
Affinity DataKd: 1.38E+3nMAssay Description:An enzyme activity assay for BLVRB was conducted. Since BLVRB catalyzes the NAD(P)H dependent readuction of FMN, changes in the NAD(P)H concentration...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Human)
University of Washington Through Its Center For Commercialization
US Patent
University of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.07E+4nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Human)
University of Washington Through Its Center For Commercialization
US Patent
University of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.31E+4nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Briefly, the HDAC8 enzyme was diluted 20 times. Then, 10 μL of HDAC8 solution was mixed with compounds at various concentrations (50 μL). T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.2Assay Description:Briefly, the assay was performed in 96-well plates in 50 mm PBS, pH 7.2 as the assay buffer, at 37 °C. The APN solution was mixed with compounds at v...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of Yersinia pestis topoisomerase 1-mediated relaxation of supercoiled plasmid DNA by agarose gel electrophoresisMore data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 1 hit for monomerid = 50061625
Found 1 hit for monomerid = 50061625
ITC DataΔG°: -8.00kcal/mole −TΔS°: -3.51kcal/mole ΔH°: -4.49kcal/mole logk: 7.40E+5
T: 25.00°C
T: 25.00°C
 3D Structure (crystal)
3D Structure (crystal)