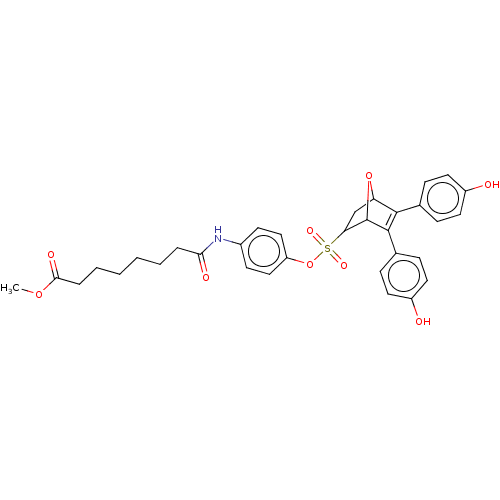
BDBM50094708 CHEMBL3589347
SMILES COC(=O)CCCCCCC(=O)Nc1ccc(OS(=O)(=O)C2CC3OC2C(=C3c2ccc(O)cc2)c2ccc(O)cc2)cc1
InChI Key InChIKey=JUOYMLJPOYQJRY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50094708
Found 6 hits for monomerid = 50094708
TargetEstrogen receptor(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human ER-alpha ligand binding domain after 2 hrs by competitive fluorometric binding assayMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Antagonist activity at ER-beta (unknown origin) transfected in HEK293T cells assessed as inhibition of transcriptional activity after 24 hrs by lucif...More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 534nMAssay Description:Inhibition of human recombinant HDAC1 after 15 mins by fluorogenic assayMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataKi: 756nMAssay Description:Binding affinity to human ER-beta ligand binding domain after 2 hrs by competitive fluorometric binding assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 6(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 1.58E+3nMAssay Description:Inhibition of human recombinant HDAC6 after 15 mins by fluorogenic assayMore data for this Ligand-Target Pair
TargetEstrogen receptor(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 1.08E+4nMAssay Description:Antagonist activity at ER-alpha (unknown origin) transfected in HEK293T cells assessed as inhibition of transcriptional activity after 24 hrs by luci...More data for this Ligand-Target Pair