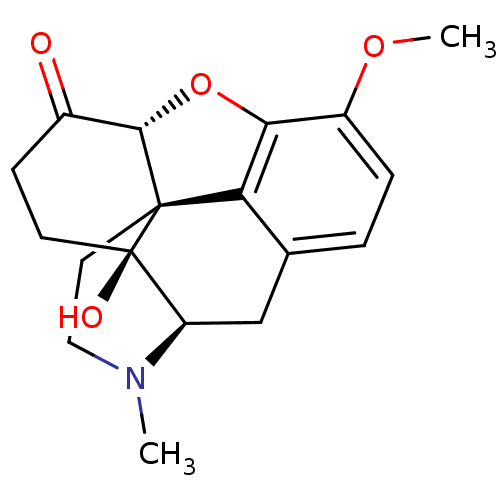
BDBM50370595 OXYCODONE::Oxycontin::US1184575, oxycodone::US9233167, Oxycodone
SMILES CN1CC[C@]23c4c5ccc(c4O[C@H]2C(=O)CC[C@]3([C@H]1C5)O)OC
InChI Key InChIKey=BRUQQQPBMZOVGD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 32 hits for monomerid = 50370595
Found 32 hits for monomerid = 50370595
TargetMu-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes after 60 mins by liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]-DAMGO from human MOR expressed in CHOK1 cell membranes incubated for 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]-DAMGO from human MOR expressed in CHOK1 cell membranes incubated for 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]-DAMGO from human MOR expressed in CHO-K1 cell membranes incubated for 60 mins measured by MicroBeta scintillation counter methodMore data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Agonist activity at human MOR expressed in CHO-K1 cells assessed as reduction in forskolin-induced cAMP production incubated for 45 mins by by HTRF a...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Agonist activity at human MOR expressed in CHOK1 cells assessed as stimulation of cAMP accumulation incubated for 45 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Agonist activity at human MOR expressed in CHOK1 cells assessed as stimulation of cAMP accumulation incubated for 45 mins by HTRF assayMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataEC50: 25nMAssay Description:Agonist activity at MOR in Wistar rat brain membranes after 60 mins by [35S]-GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors can use 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, CT), with 5 mg mem...More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 43.6nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 133nM ΔG°: -9.37kcal/moleT: 2°CAssay Description:Briefly, serial dilutions of the test compounds were placed in a 96-well plate to which were added SPA beads, membrane and radioligand. The assay con...More data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Agonist activity at mouse MOR assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by cell based TR-FRET assayMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Guinea pig)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 325nMAssay Description:Displacement of [3H]HS-665 from KOR in guinea pig brain membranes after 60 mins by liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 478nMT: 2°CAssay Description:Briefly, suspensions of cells expressing either the mu, kappa or delta opioid receptors were prepared in buffer containing 0.5 mM isobutyl-methyl xan...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 487nMAssay Description:Displacement of [3H]Ile5,6-deltorphin-2 from DOR in Wistar rat brain membranes after 60 mins by liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 500nMAssay Description:Agonist activity at human MOR assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by cell based TR-FRET assayMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.09E+3nMAssay Description:Displacement of [3H[Ile5,6]-deltorphin II from delta opioid receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.66E+3nMAssay Description:Displacement of [3H]U-69593 from kappa opioid receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataEC50: 4.00E+3nMAssay Description:Agonist activity at human DOR assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by cell based TR-FRET assayMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor in rat brain membraneMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1/2(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]WIN-55,212-2 from CB1R/CB2R in Wistar rat brain membranes after 60 mins by liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-(+)-pentazocine from human sigma-1 receptor transfected in HEK293 membranes incubated for 120 mins measured by microBeta scintil...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Esteve Pharmaceuticals
Curated by ChEMBL
Esteve Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells at -80 mV holding potential by whole cell patch clamp assayMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Guinea pig)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membraneMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of 3H](+)-pentazocine from human sigma1 receptor expressed in HEK293 cell membranes incubated for 120 mins by liquid scintillation count...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1/2(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]JWH-018 from CB1R/CB2R in Wistar rat brain membranes after 60 mins by liquid scintillation analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-(+)-pentazocine from human sigma-1 receptor expressed in HEK293 membranes incubated for 120 mins by liquid scintillation countin...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rat)
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Biological Research Centre of The Hungarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H][Ile5,6]deltorphin2 from delta opioid receptor in rat brain membraneMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+4nMAssay Description:Agonist activity at mouse KOR assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by cell based TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+4nMAssay Description:Agonist activity at human KOR assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by cell based TR-FRET assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electric eel)
University of Innsbruck and Center For Molecular Biosciences Innsbruck-Cmbi
Curated by ChEMBL
University of Innsbruck and Center For Molecular Biosciences Innsbruck-Cmbi
Curated by ChEMBL
Affinity DataIC50: 7.62E+4nMAssay Description:Inhibition of electric eel AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of human OCT1 expressed in HEK293 cells assessed as reduction in ASP+ substrate uptake by microplate reader based analysisMore data for this Ligand-Target Pair