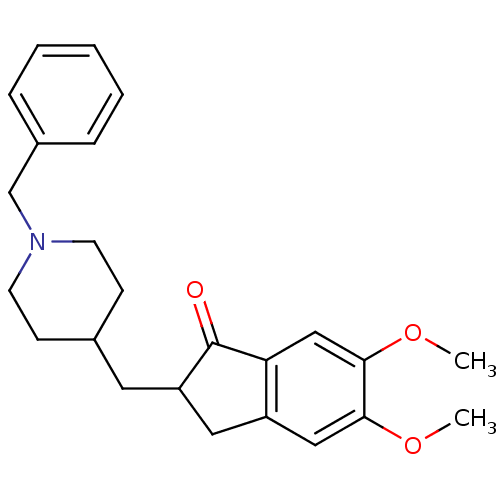
BDBM8960 (+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-indan-1-one::2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one::Aricept::Aricept odt::CHEMBL1678::CHEMBL2337271::CHEMBL502::DONEPEZIL HYDROCHLORIDE::Donepezil::Donepzil::E2020::US8999994, Donepezil::US9346818, DPH::US9586925, Donepezil::US9663465, Donepezil
SMILES COc1cc2c(cc1OC)C(=O)[C@@H](C2)CC3CCN(CC3)Cc4ccccc4
InChI Key InChIKey=ADEBPBSSDYVVLD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 700 hits for monomerid = 8960
Found 700 hits for monomerid = 8960
Affinity DataIC50: 1.70nMAssay Description:In vitro inhibitory effect on rat AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of rat cortex homogenate acetylcholinesterase using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of acetylcholinesterase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nM ΔG°: -11.6kcal/molepH: 8.0 T: 2°CAssay Description:Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ...More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Inhibition of fetal Bovine serum AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of AChE (unknown origin) incubated for 30 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition measured after 3 ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate incubated for 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:In vitro inhibitory activity against acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Inhibition of AChE in mouse cerebral cortex using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 5 mins prior to substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of AChE in mouse brain homogenate using acetylthiocholine as substrate preincubated for 300 secs followed by substrate addition by Ellman'...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of Torpedo californica AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of rat cortex AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibitory concentration in vitro and ex vivo for anti-AChE activity in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibitory activity against acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human recombinant AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human erythrocyte AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate by Ellman' methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brainMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human AChE using ATCI as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins by spectro...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of AChE-induced amyloid beta aggregationMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human recombinant AChE pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.16nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Inhibition of Electrophorus electricus AchE using acetylthiocholine iodide as substrate preincubated for 10 mins measured after 15 mins of substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine as substrate by UV-visible spectrophotometric Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate peincubated for 5 mins followed by substrate addition by DTNB-reagen...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured after 10...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of AchE (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:Inhibition of recombinant AChE in human erythrocytes using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of AChE in rat cortex preincubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)