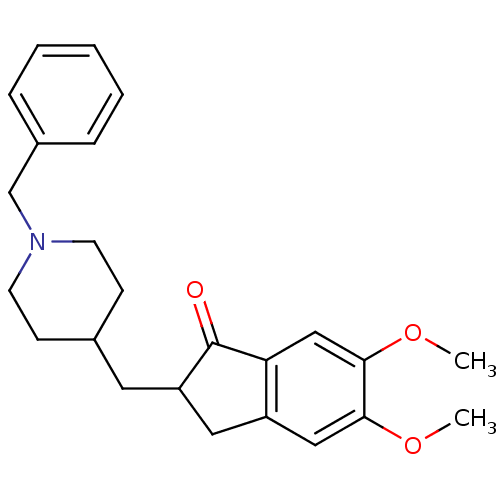
BDBM8960 (+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-indan-1-one::2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one::Aricept::Aricept odt::CHEMBL1678::CHEMBL2337271::CHEMBL502::DONEPEZIL HYDROCHLORIDE::Donepezil::Donepzil::E2020::US8999994, Donepezil::US9346818, DPH::US9586925, Donepezil::US9663465, Donepezil
SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC
InChI Key InChIKey=ADEBPBSSDYVVLD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 210 hits for monomerid = 8960
Found 210 hits for monomerid = 8960
Affinity DataKi: 640nMAssay Description:Inhibition of Equine ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataKi: 2.14E+3nMAssay Description:Non-competitive inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.32E+3nMAssay Description:Inhibition assay using AChE and BuChE.More data for this Ligand-Target Pair
Affinity DataKi: 1.25E+4nMAssay Description:Non competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.42E+3nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob...More data for this Ligand-Target Pair
Affinity DataIC50: 4.15E+3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.15E+3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.52E+4nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.37E+3nMAssay Description:Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me...More data for this Ligand-Target Pair
Affinity DataIC50: 6.41E+3nMAssay Description:Inhibition of BuChE in equine serum using butyrylthiocholine chloride as substrate preincubated with enzyme for 10 mins prior to substrate challenge ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.37E+3nMAssay Description:Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me...More data for this Ligand-Target Pair
Affinity DataIC50: 1.82E+3nMAssay Description:Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.92E+3nMAssay Description:Inhibition of BuChE (unknown origin) after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.38E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide substrate by Ellman method based spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.46E+3nMAssay Description:Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Inhibition of BuChE in horse serum pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.91E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate incubated for 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibition of recombinant human BuChE expressed in HEK293 cells preincubated for 15 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.31E+3nMAssay Description:Inhibition of equine serum butyrylcholine esterase incubated for 15 mins using S-butyrylthiocholine chloride substrate by colorimetric Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of equine serum BuChE pre-incubated for 5 mins before butyrylthiocholine iodide substrate by Ellman' methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Inhibition of equine serum BuChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of human serum BuChE by spectrophotometric Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+4nMAssay Description:Inhibition of human BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of horse serum butyrylcholine esterase in presence of acetylcholine substrate by chemiluminescent assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 8.71E+3nMAssay Description:Inhibition of equine serum BuChE using DTNB as substrate incubated for 5 mins prior to substrate addition measured after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.42E+3nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.62E+3nMAssay Description:Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
Affinity DataIC50: 540nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.74E+3nMAssay Description:Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.79E+3nMAssay Description:Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to...More data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min...More data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+3nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocoline iodide as substrate incubated for 20 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.36E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2...More data for this Ligand-Target Pair
Affinity DataIC50: 3.36E+3nMAssay Description:Inhibition of equine BCHE preincubated for 6 mins followed by addition of S-butyrylcholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.76E+3nMAssay Description:Inhibition of human serum BCHE preincubated for 6 mins followed by addition of S-butyrylcholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.42E+3nMAssay Description:Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human BChE after 20 mins using butyrylthiocholine iodide as a substrate by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of human BChE using butyrylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of equine BuChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of equine serum BuchE using butyrylthiocholine iodide as substrate preincubated for 10 mins measured after 15 mins of substrate addition b...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Concentration required to inhibit 50% of Butyrylcholinesterase obtained from human serum was determined in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibitory concentration against Butyrylcholinesterase (BuChE) from human serumMore data for this Ligand-Target Pair