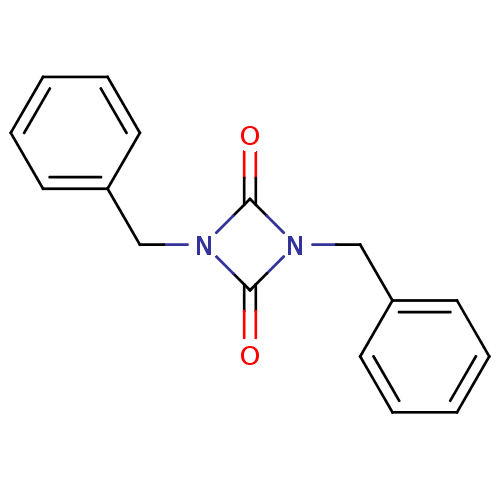
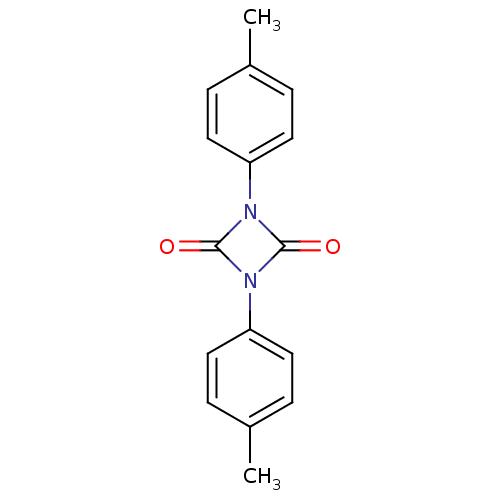
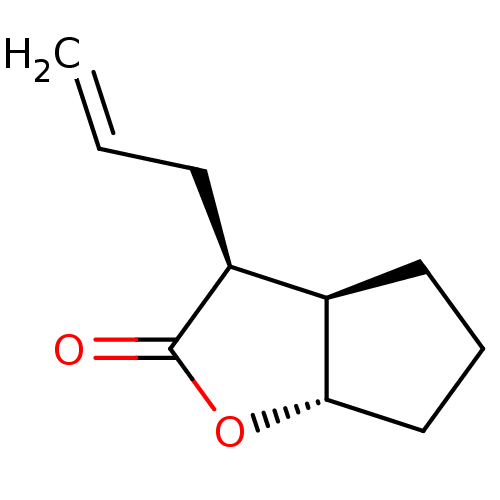
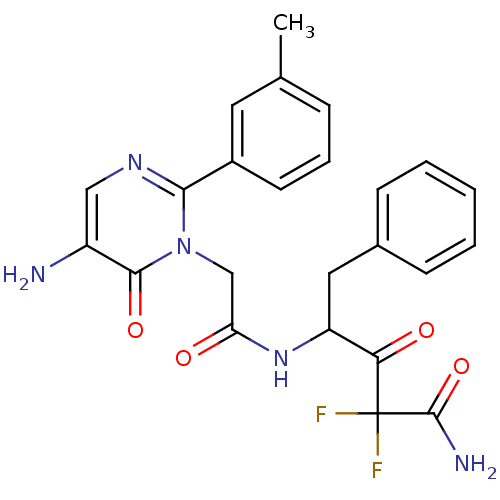
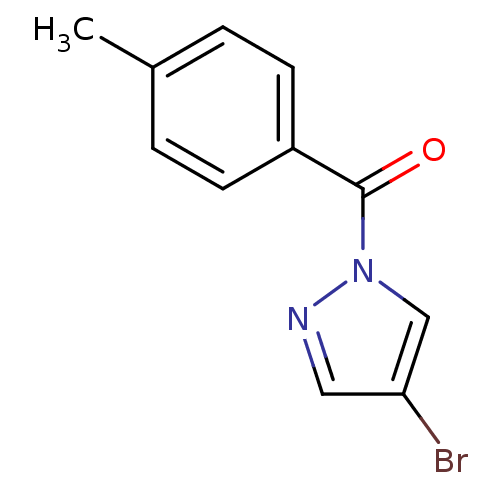
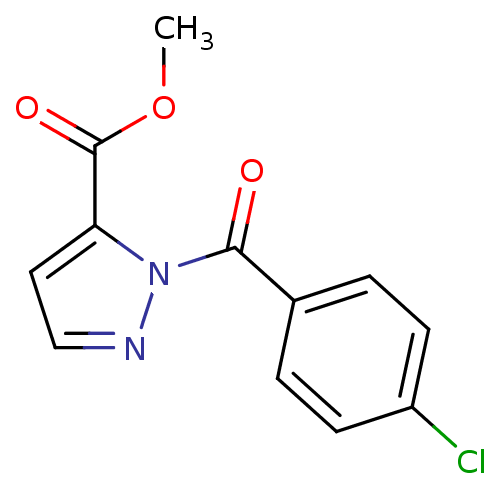
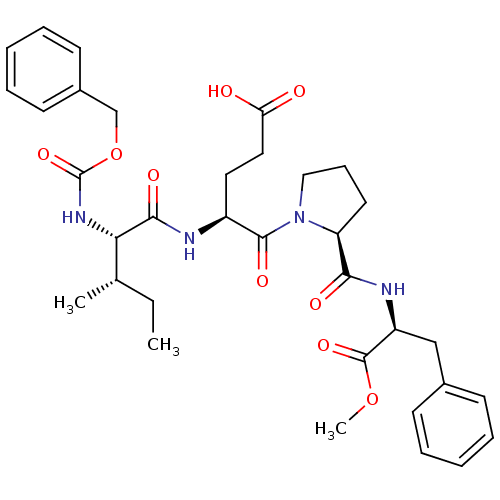
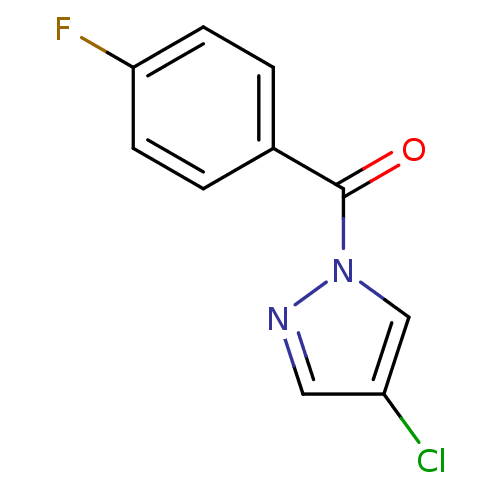
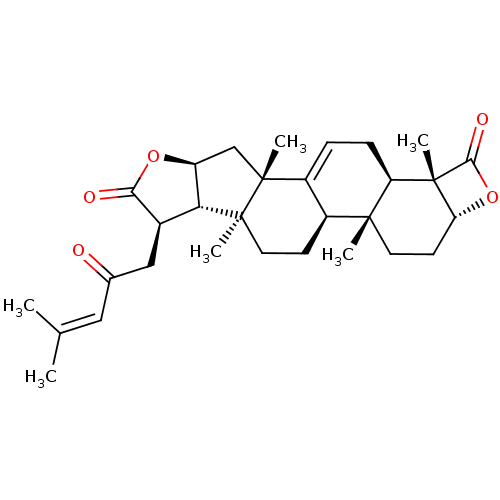
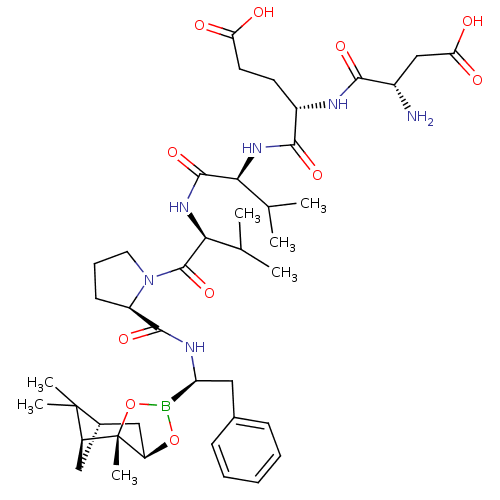
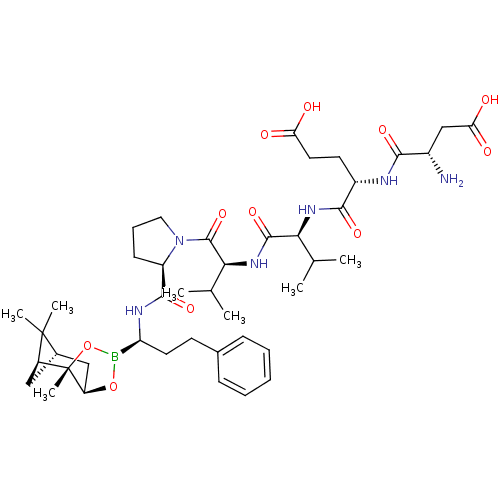
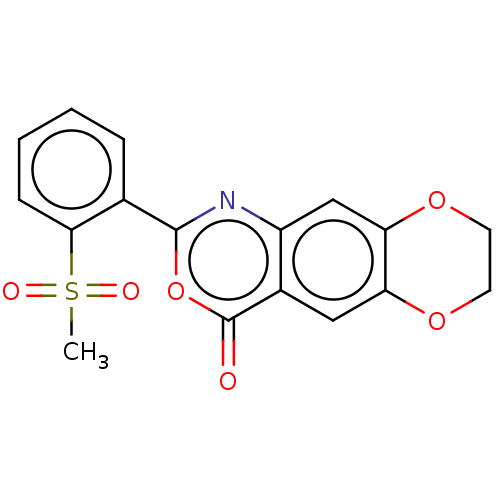
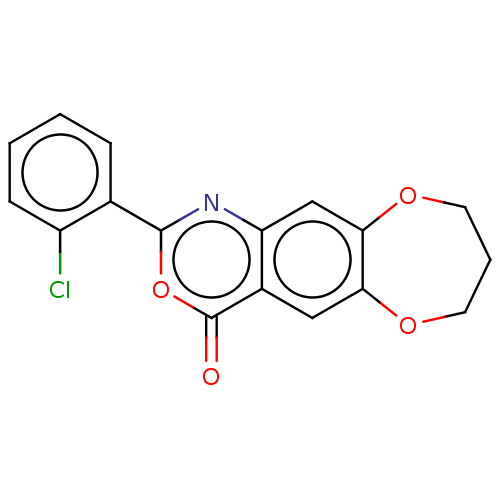
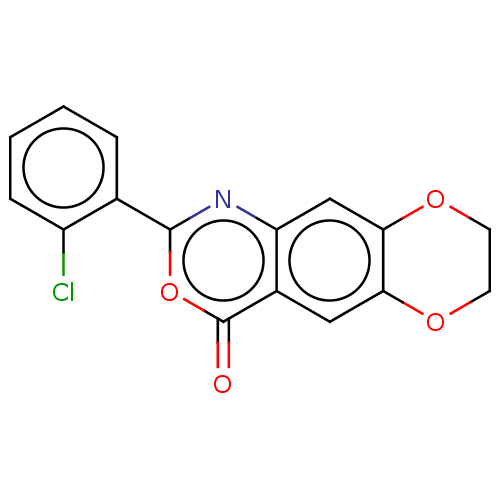
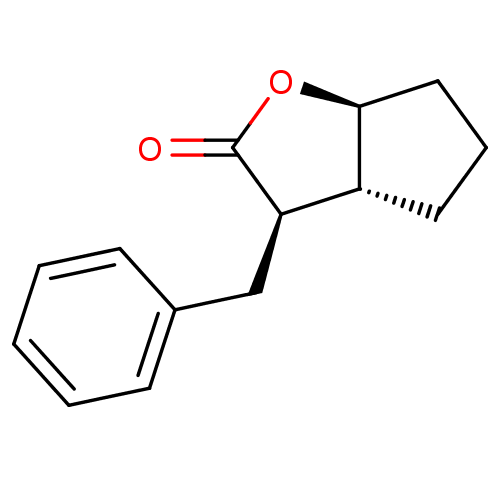
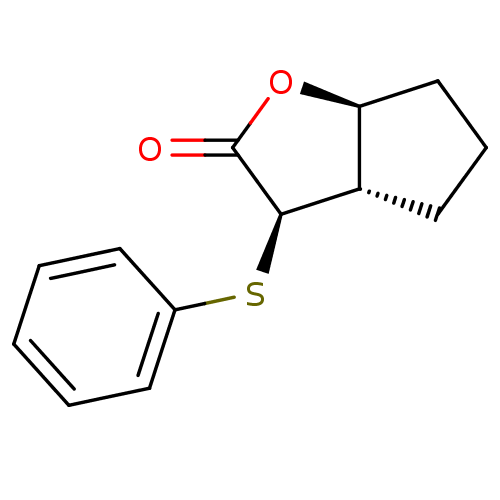
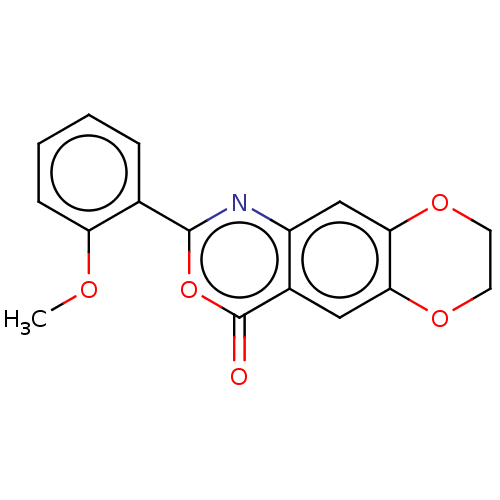
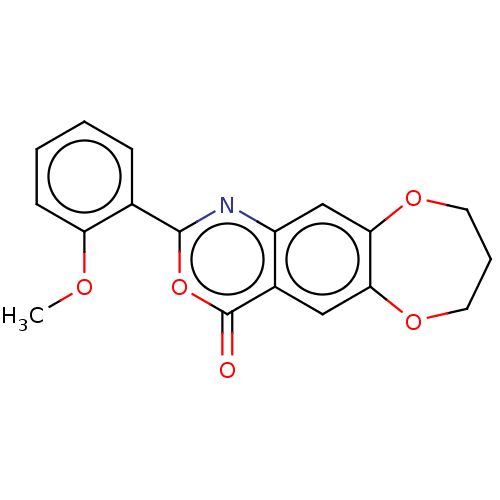
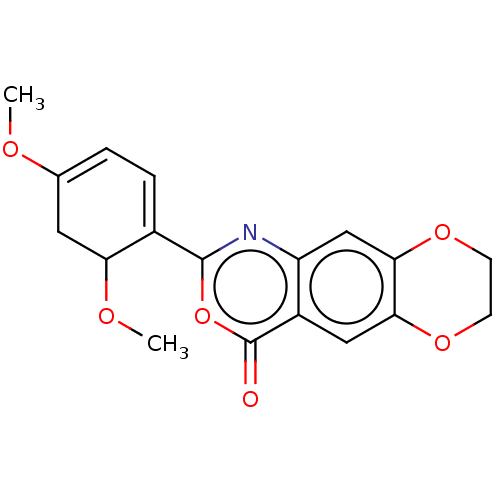
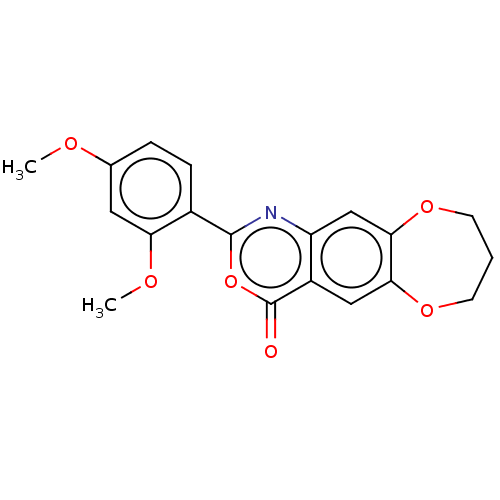
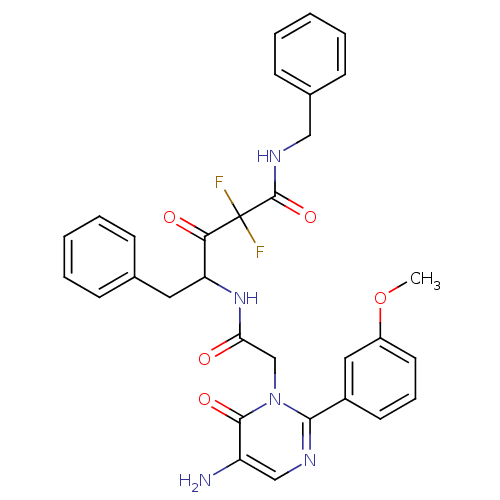
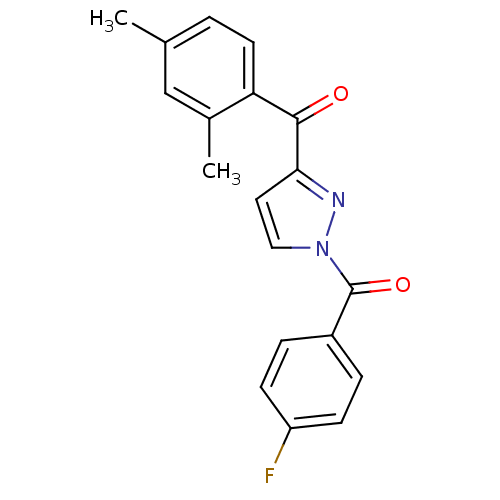
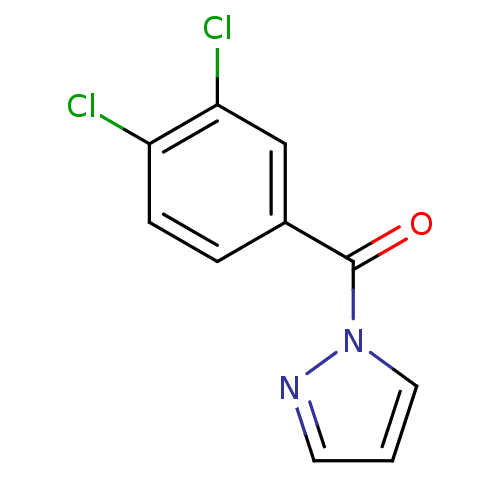
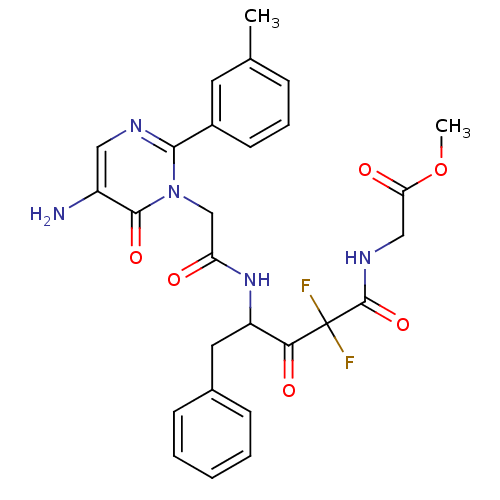
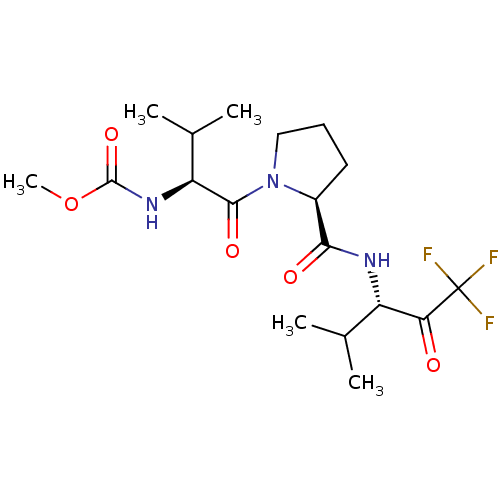
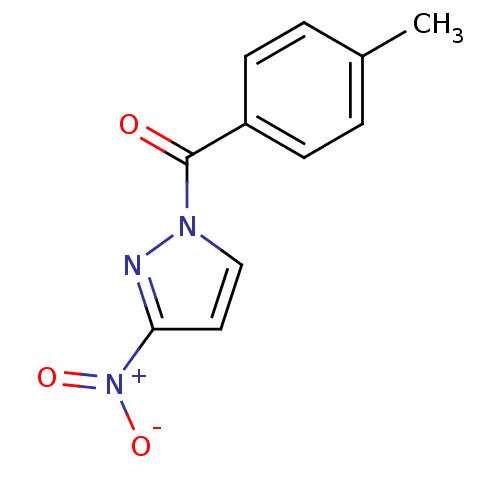
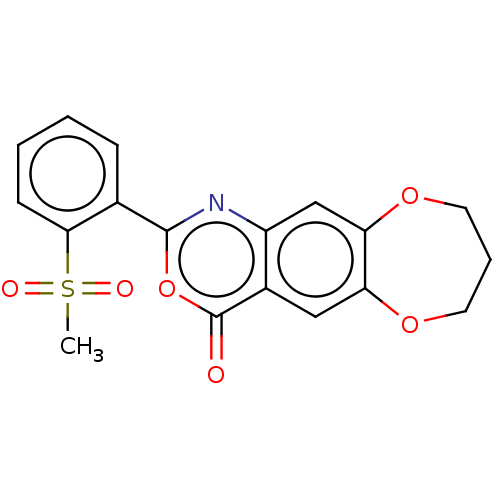
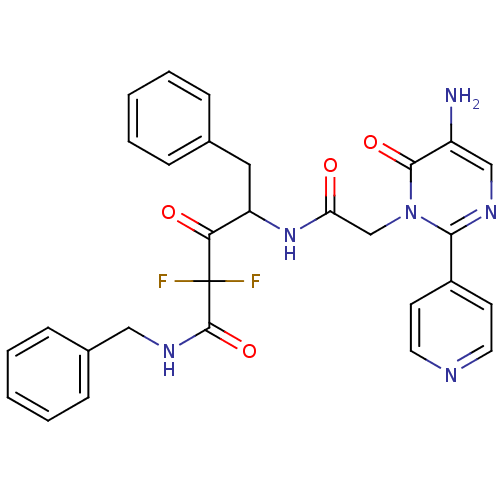
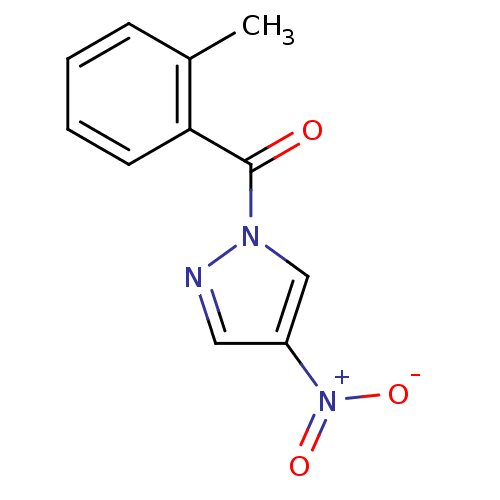
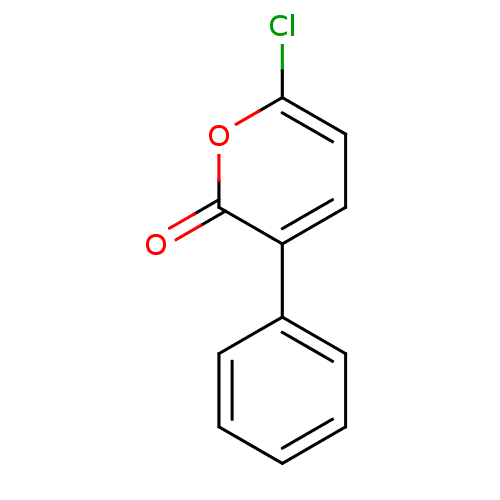
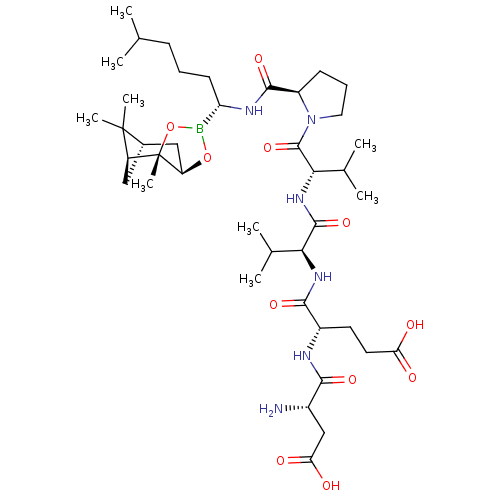
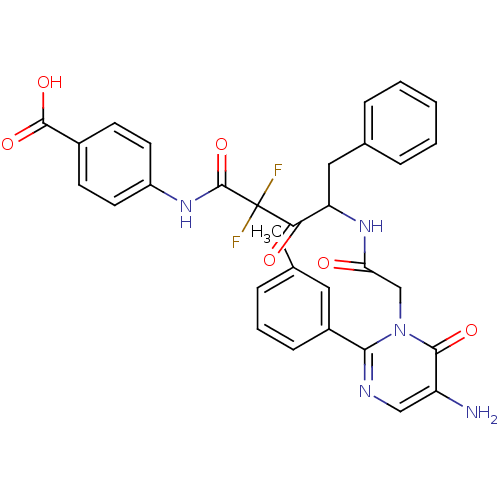
Affinity DataIC50: 0.690nMAssay Description:Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsinMore data for this Ligand-Target Pair
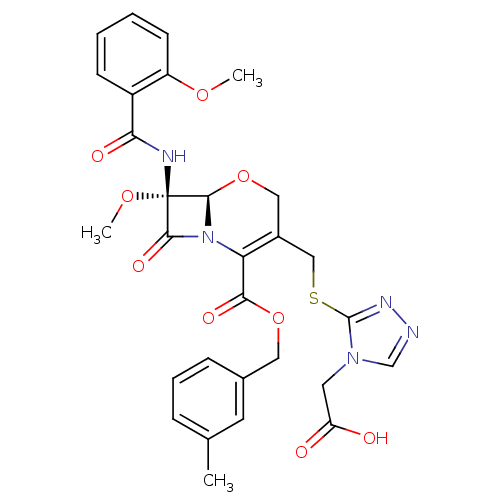
Affinity DataIC50: 2.40nMAssay Description:Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsinMore data for this Ligand-Target Pair
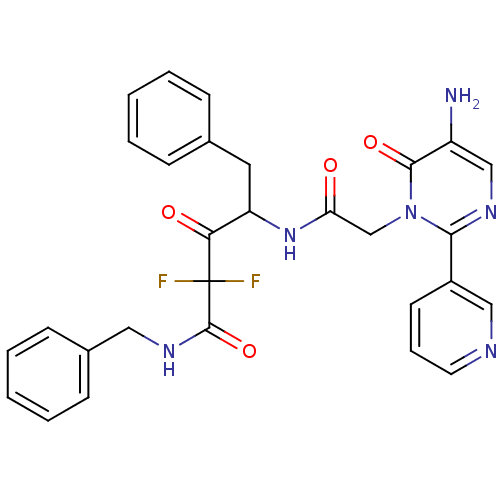
Affinity DataIC50: 7nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
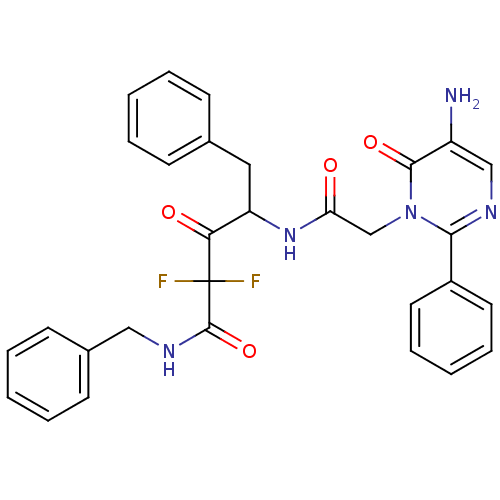
Affinity DataKi: 9.36nMAssay Description:inhibitory activity against alpha chymotrypsin from bovine pancreas.More data for this Ligand-Target Pair
Affinity DataKi: 9.36nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 12.6nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Compound was evaluated for inhibitory activity against Bovine ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibitory concentration against Human pancreatic Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Tested for inhibitory activity against bovine pancreatic chymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:The compound was evaluated for the binding affinity against bovine pancreatic chymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibitory concentration against Human pancreatic Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibitory concentration against Human pancreatic Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Inhibitory concentration against Human pancreatic Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataKi: <100nM ΔG°: <-41.6kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataKi: 109nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 147nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 149nMAssay Description:Binding affinity for human pancreatic ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:The compound was evaluated for the inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 171nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 177nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 200nM ΔG°: -39.8kJ/molepH: 7.2 T: 2°CAssay Description:Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi...More data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:In vitro binding affinity towards alpha-chymotrypsin from bovine pancreas.More data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibitory concentration against Human pancreatic Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 282nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 295nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibitory concentration against Human pancreatic Serine protease chymotrypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Compound was tested for inhibition of Serine protease chymotrypsinMore data for this Ligand-Target Pair