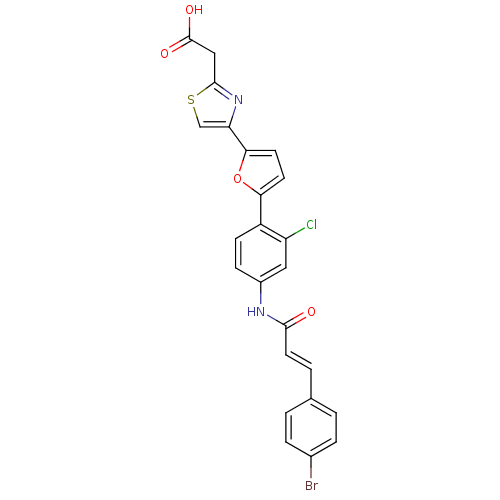
BDBM50165636 2-(4-(5-(4-(3-(4-bromophenyl)acrylamido)-2-chlorophenyl)furan-2-yl)thiazol-2-yl)acetic acid::CHEMBL197366::[4-(5-{4-[3-(4-Bromo-phenyl)-acryloylamino]-2-chloro-phenyl}-furan-2-yl)-thiazol-2-yl]-acetic acid
SMILES OC(=O)Cc1nc(cs1)-c1ccc(o1)-c1ccc(NC(=O)\C=C\c2ccc(Br)cc2)cc1Cl
InChI Key InChIKey=IIFRGJWMWZUWTR-XCVCLJGOSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50165636
Found 4 hits for monomerid = 50165636
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human heparanase assessed as reduction in basic fibroblast growth factor binding incubated for 2 hrs by microplate reader assayMore data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Inhibition of heparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3)More data for this Ligand-Target Pair