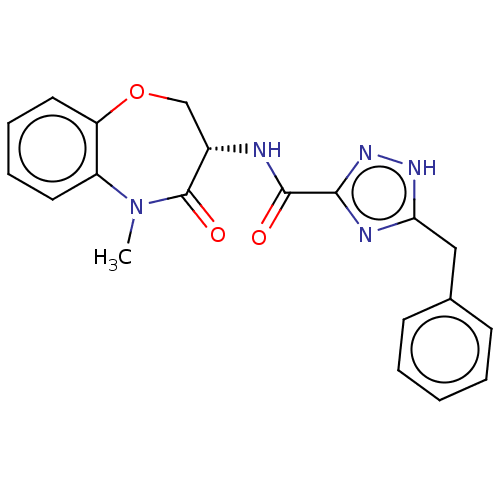
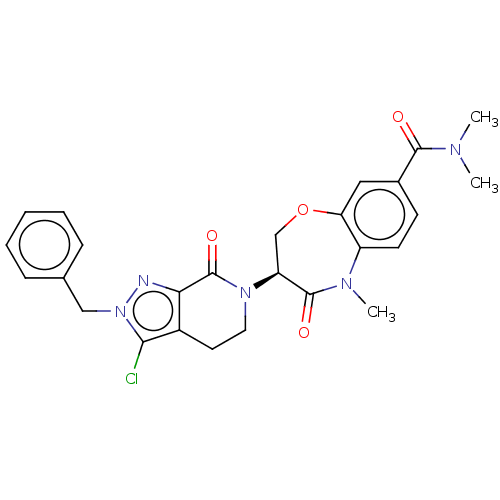
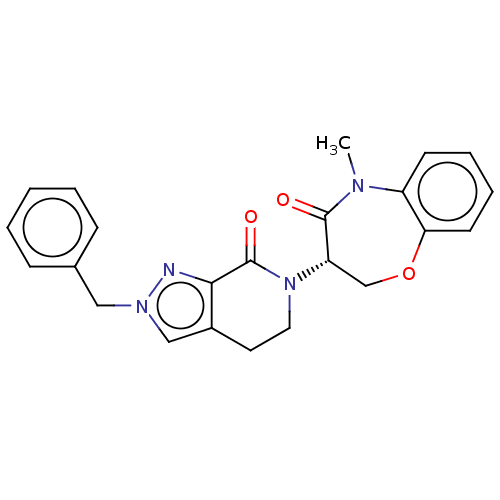
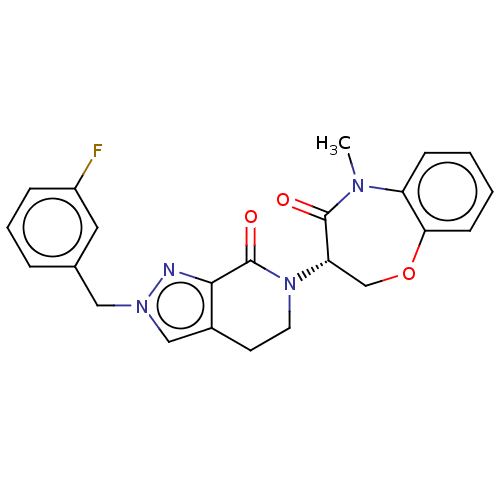
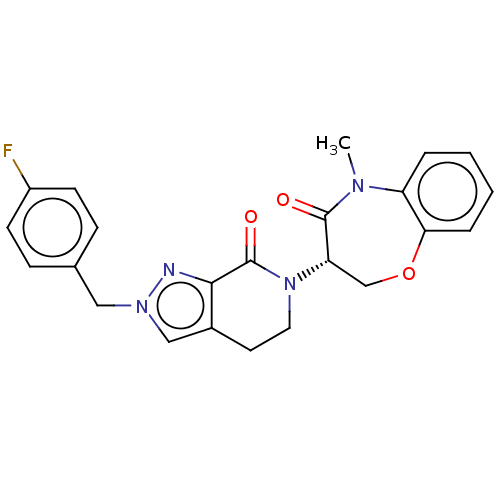
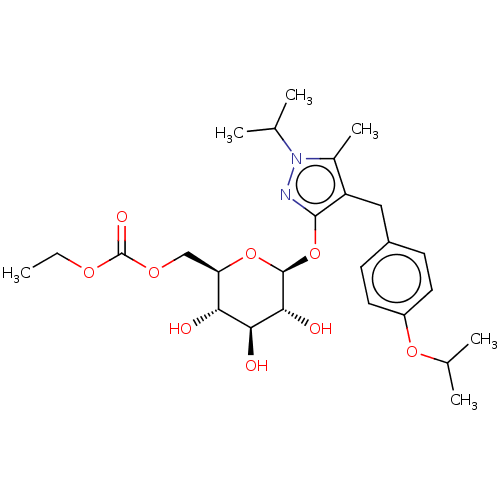
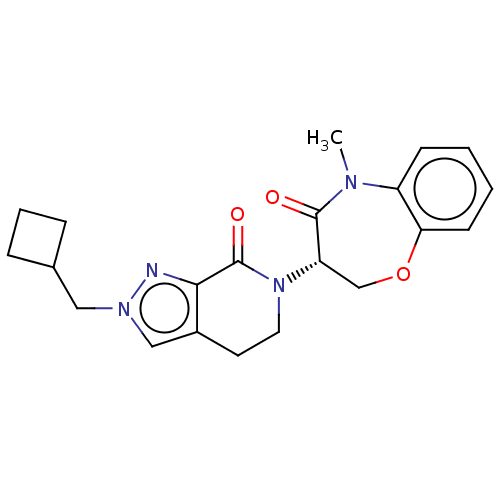
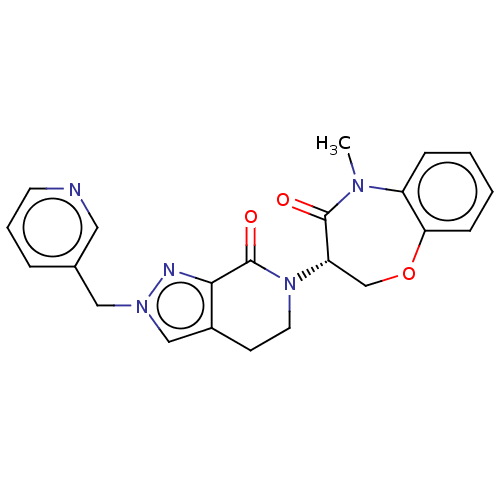
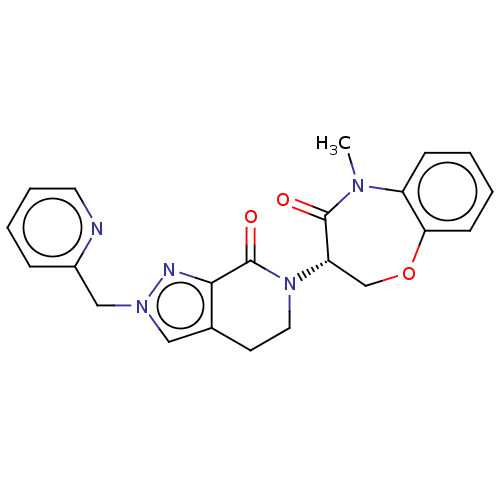
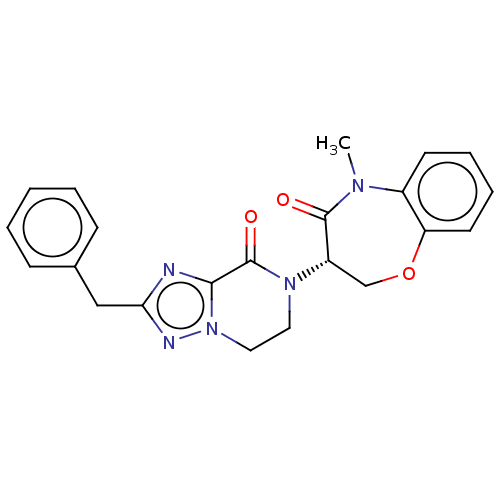
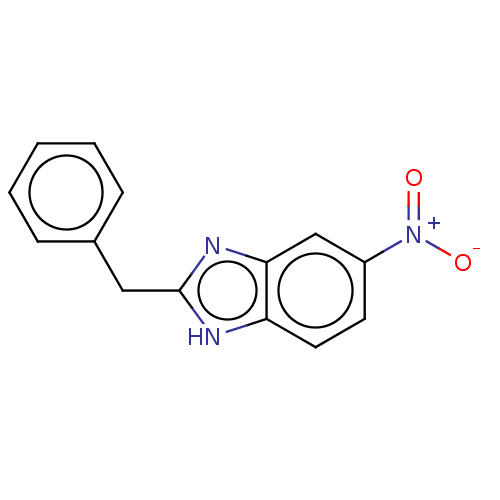
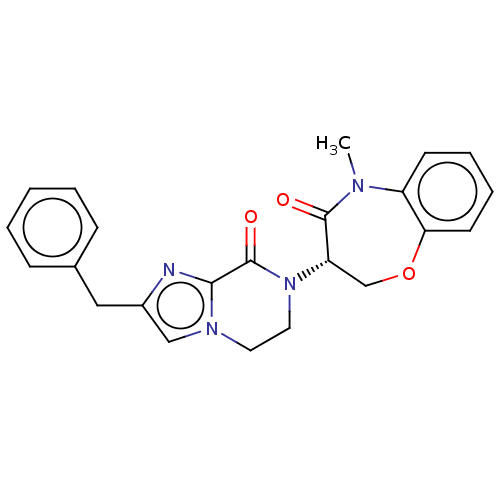
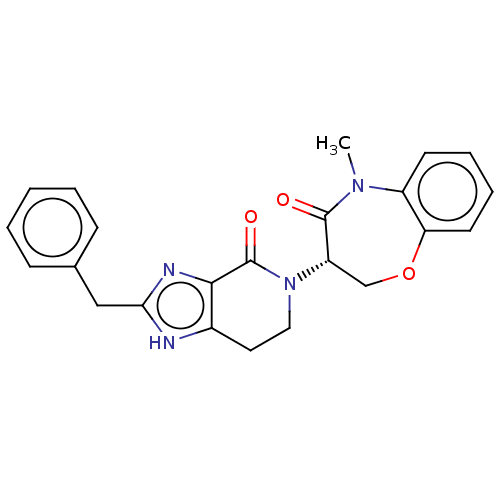
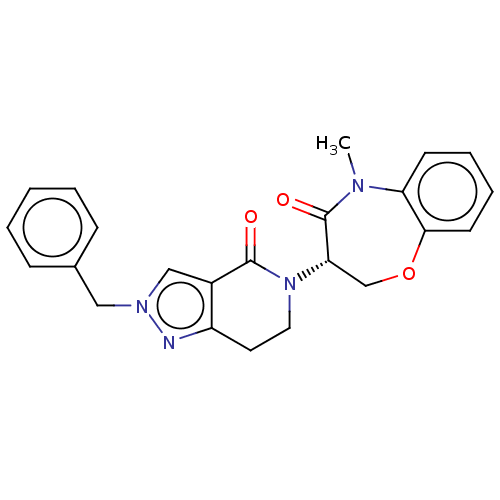
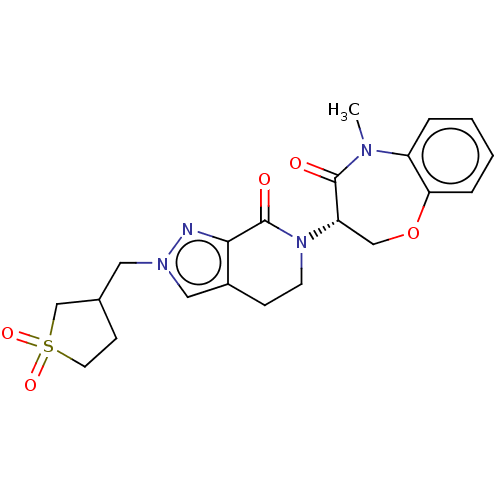
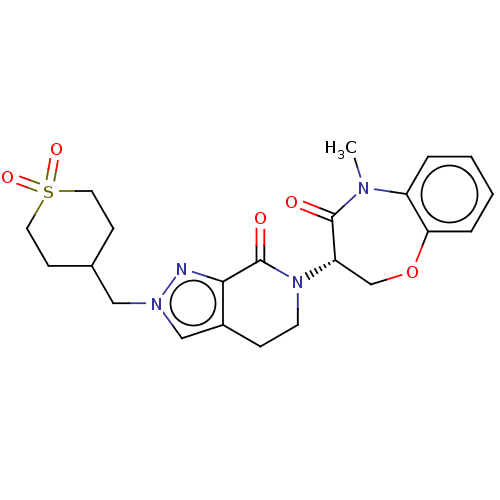
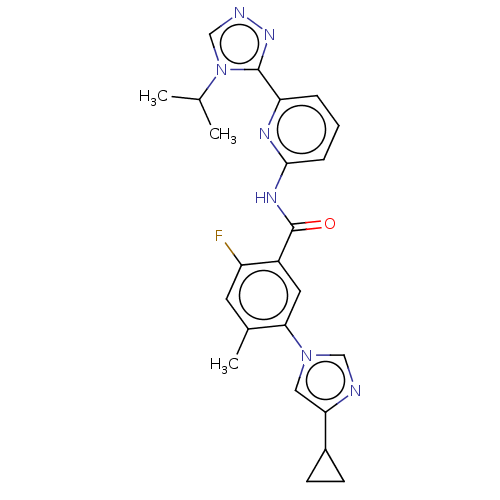
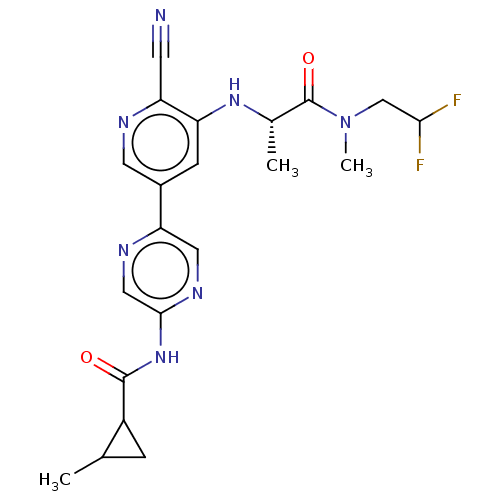
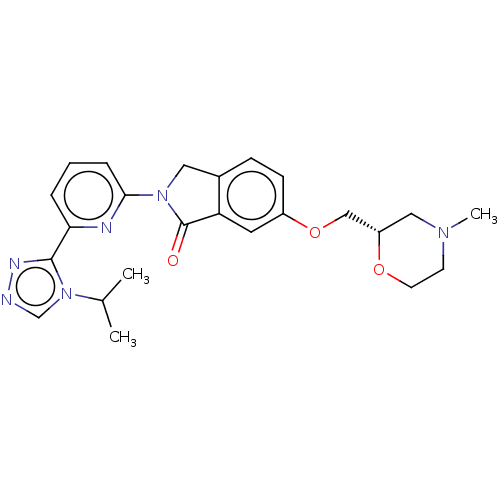
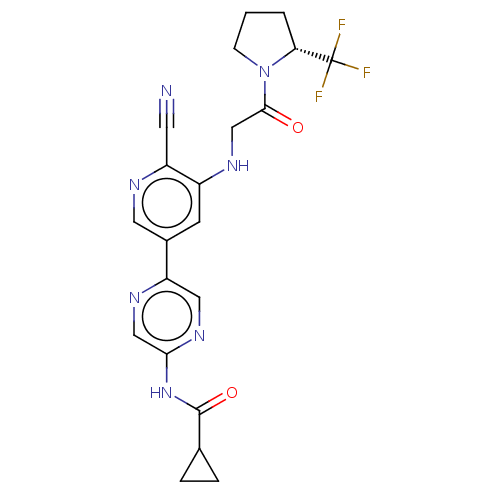
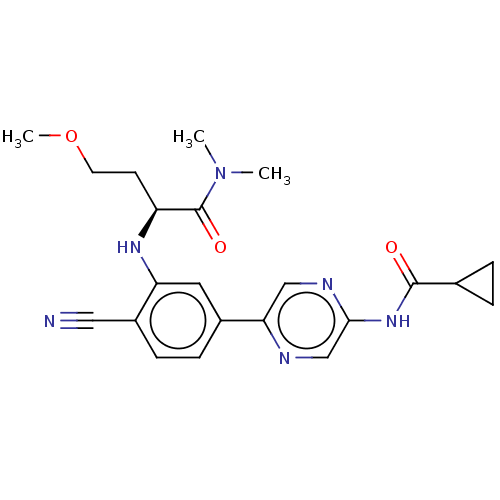
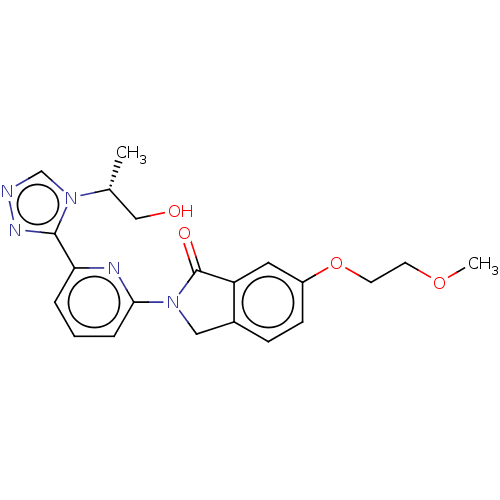
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
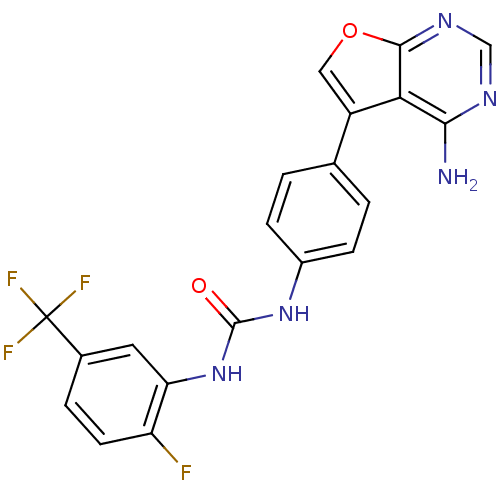
Affinity DataKi: 0.407nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
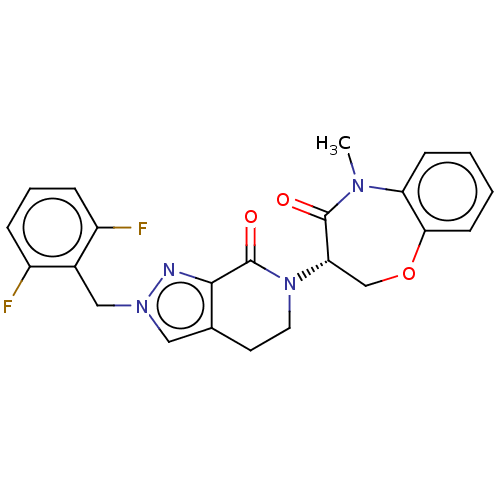
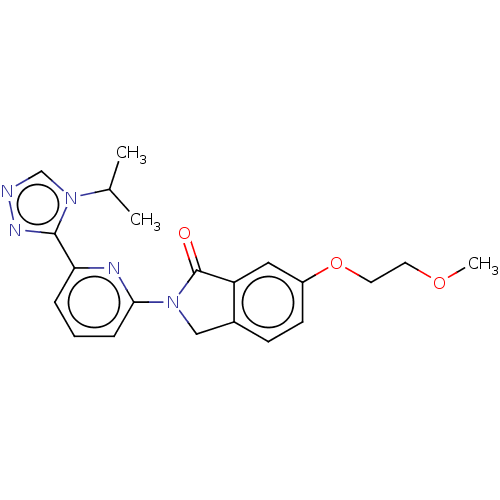
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 0.851nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
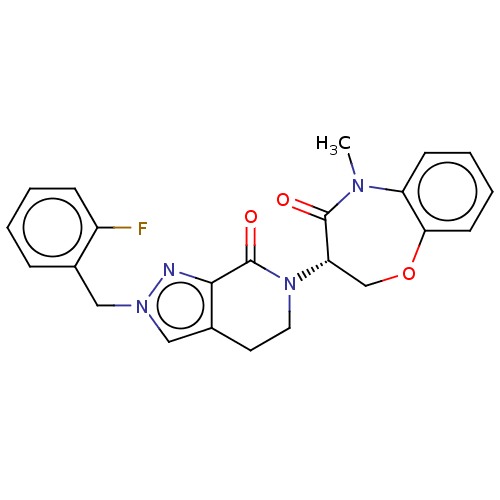
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 0.912nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
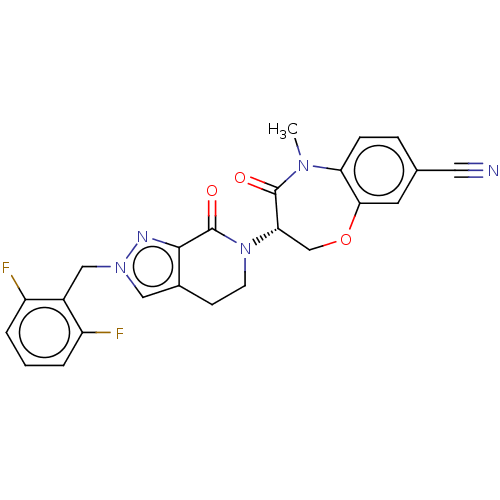
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 0.912nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 0.977nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 3.90nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 5.10nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 7.90nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Antagonist potency against adenosine A2B receptor of guinea pig thoracic aortic smooth muscleMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 34nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 55nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 63nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Mus musculus)
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 81nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 107nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 117nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 251nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 4.27E+3nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
Affinity DataKi: 4.52E+3nMAssay Description:Antagonist potency at cloned recombinant human adenosine A2B receptor transfected in CHO cells by cAMP assayMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 6.92E+3nMAssay Description:Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
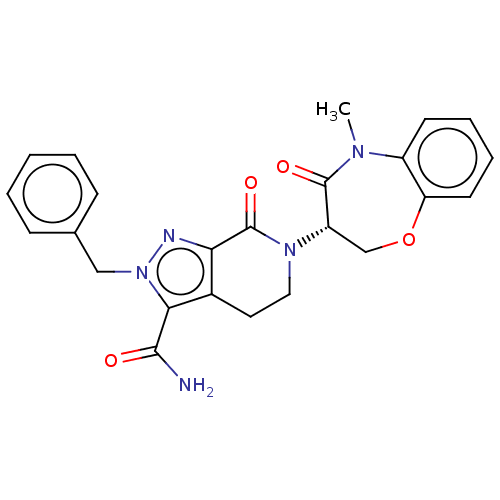
Affinity DataIC50: 1.30nMAssay Description:Inhibition of RIPK1 in human HT-29 cells assessed as decrease in TNFalpha/AT-406/zVAD-FMK-induced MLKL phosphorylation at S358 residue preincubated f...More data for this Ligand-Target Pair
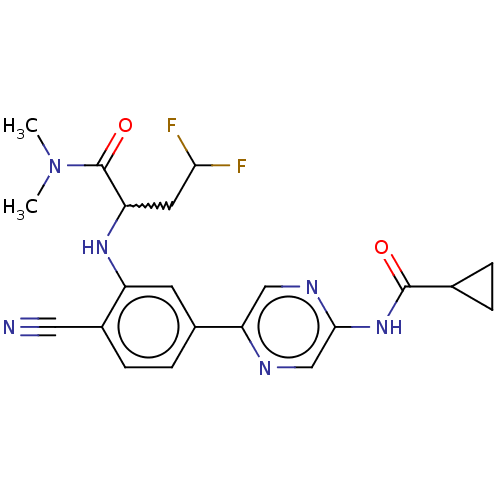
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
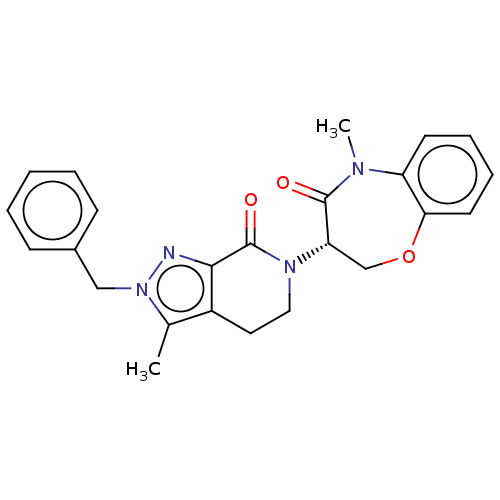
Affinity DataIC50: 2nMAssay Description:Inhibition of ASK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Mus musculus)
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Inhibition of RIPK1 in mouse L929 cells assessed as decrease in TNFalpha/zVAD-FMK-induced MLKL phosphorylation at S358 residue preincubated for 30 mi...More data for this Ligand-Target Pair
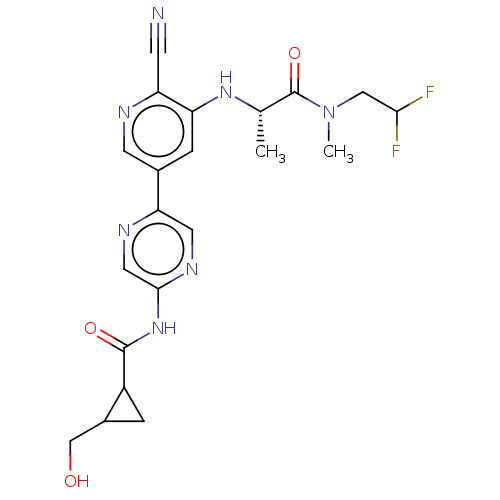
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of ASK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.16nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 3(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
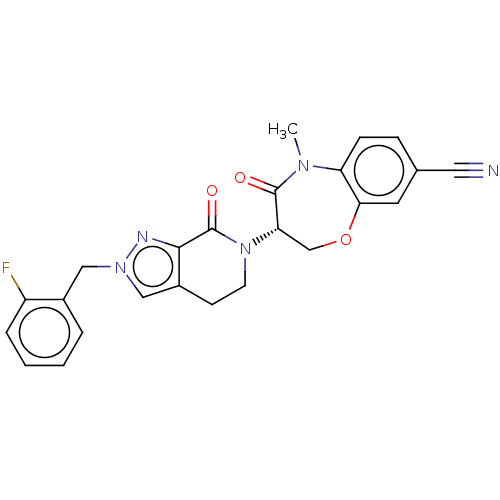
Affinity DataIC50: 4nMAssay Description:Inhibition of MKK3 (unknown origin) using [gamma-33P]-ATP after 20 mins by radiometric assayMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 3(Homo sapiens (Human))
Takeda California
Curated by ChEMBL
Takeda California
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of MKK3 (unknown origin) using [gamma-33P]-ATP after 20 mins by radiometric assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged ASK1 catalytic domain (654 to 971 residues) expressed in baculovirus infected sf21 cells using ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged ASK1 catalytic domain (654 to 971 residues) expressed in baculovirus infected sf21 cells using ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.01nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged ASK1 catalytic domain (654 to 971 residues) expressed in baculovirus infected sf21 cells using ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.31nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.31nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.31nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged ASK1 catalytic domain (654 to 971 residues) expressed in baculovirus infected sf21 cells using ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 5(Homo sapiens (Human))
Takeda Research In California
Curated by ChEMBL
Takeda Research In California
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged ASK1 catalytic domain (654 to 971 residues) expressed in baculovirus infected sf21 cells using ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:TBK1 inhibition is determined using a 384 well μlate format in buffer containing 20 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 0.01% Brij-L23, 1 ...More data for this Ligand-Target Pair

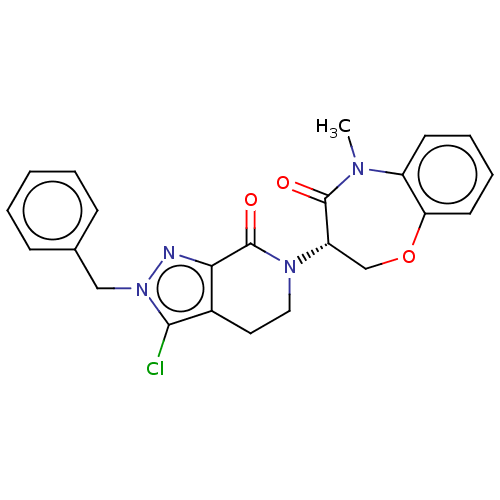
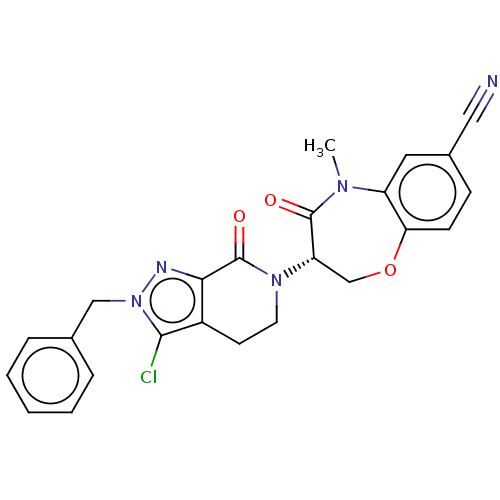
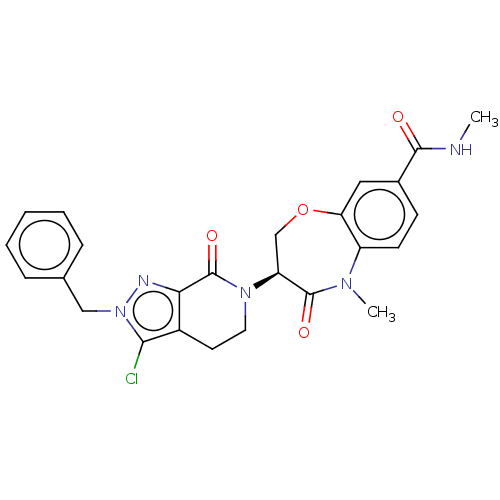
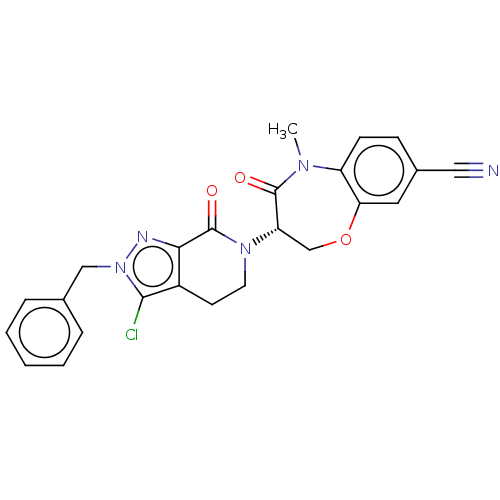
 3D Structure (crystal)
3D Structure (crystal)