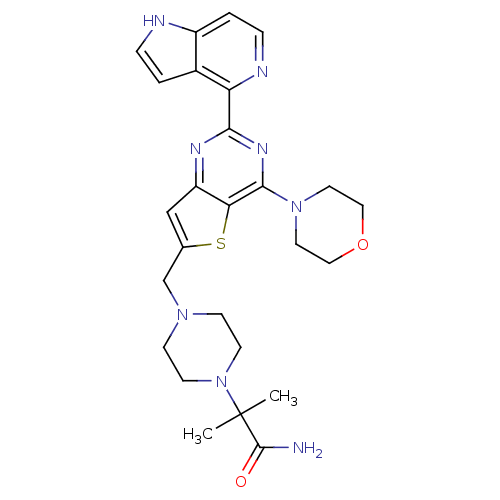
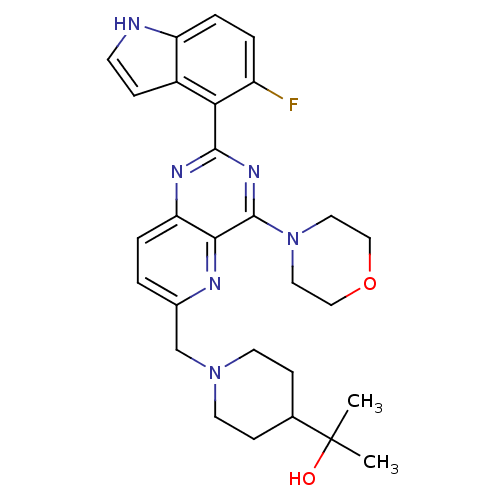
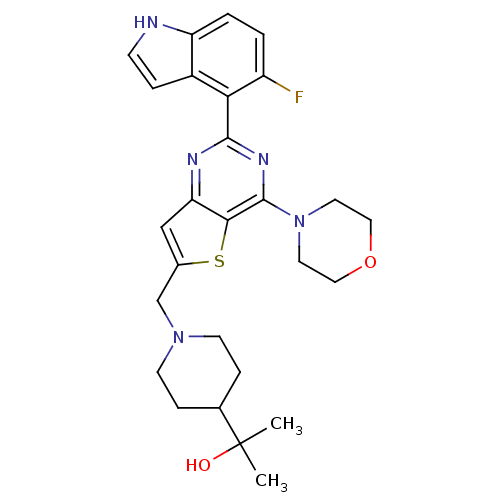
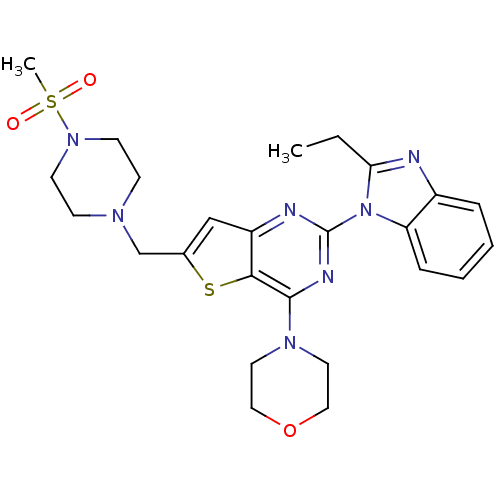
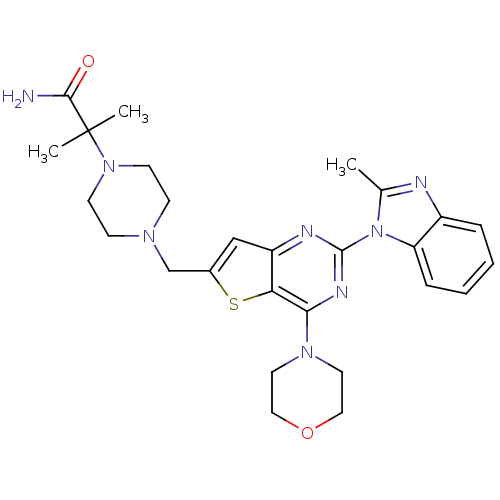
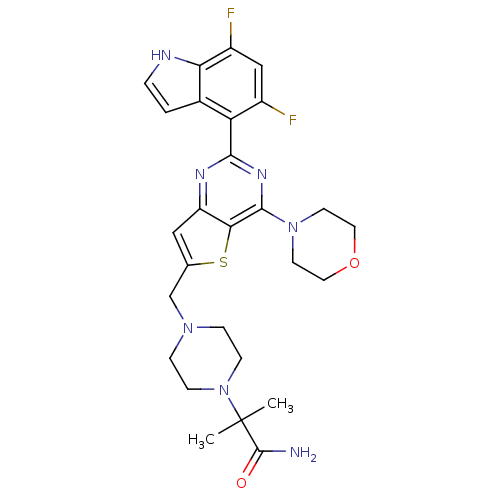
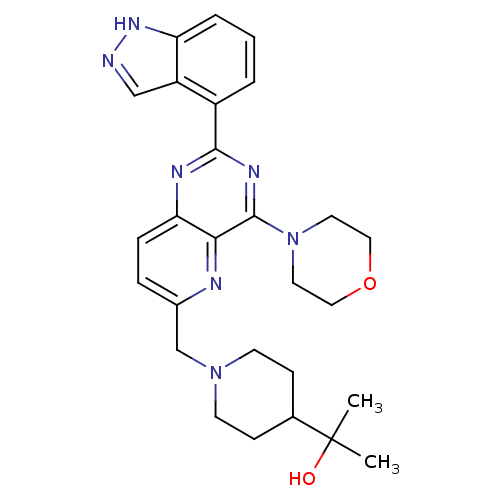
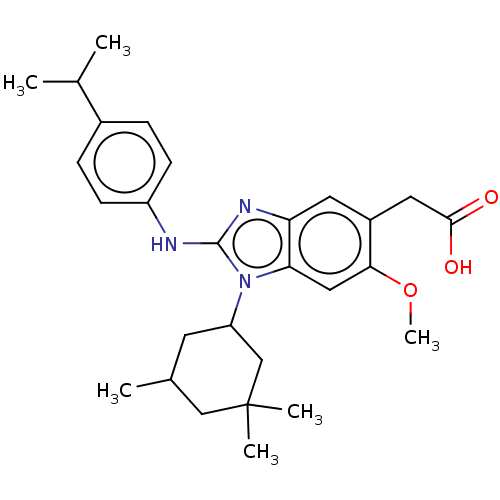
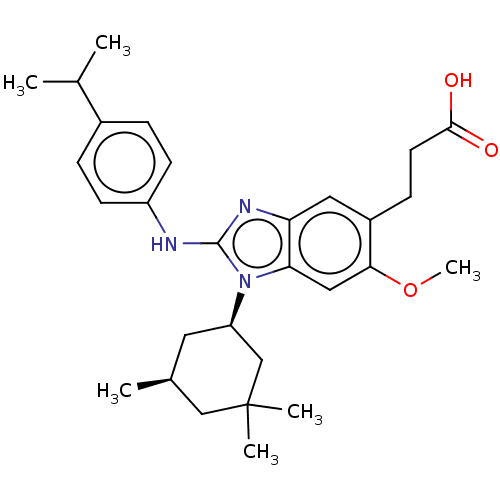
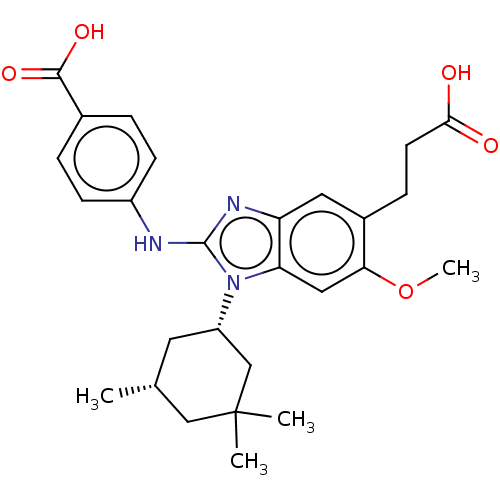
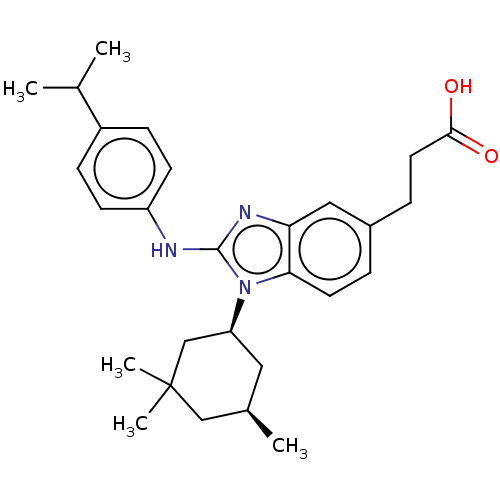
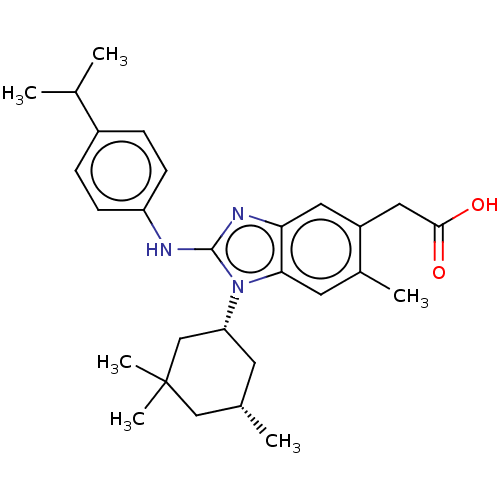
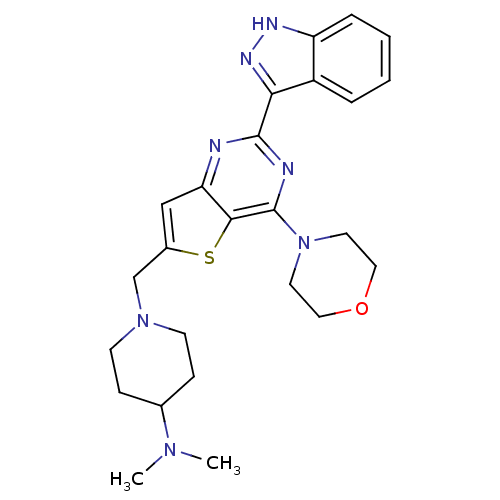
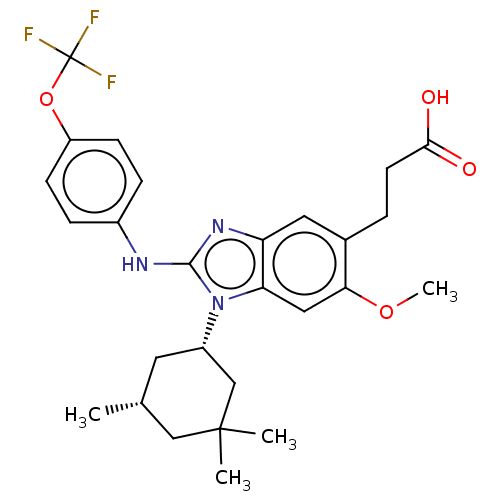
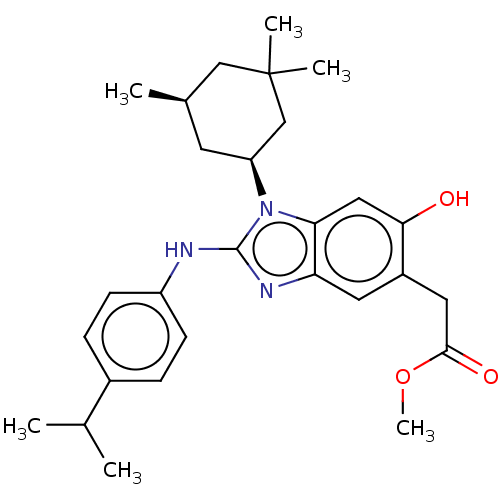
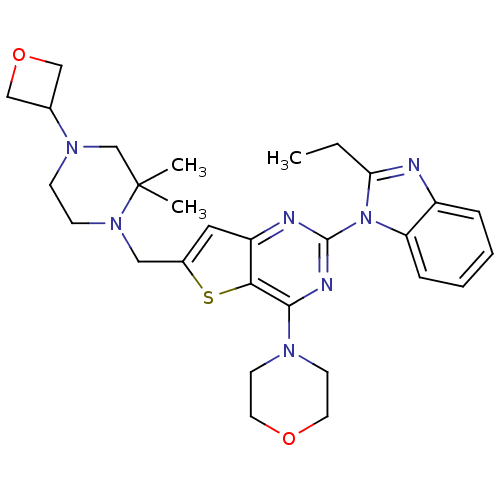
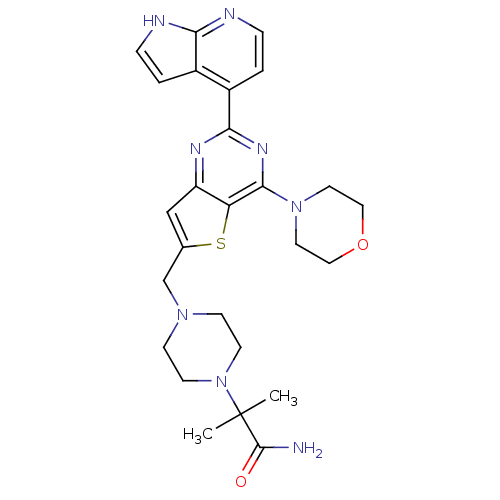
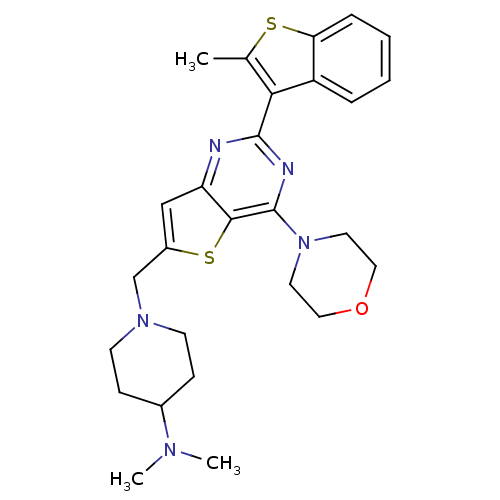
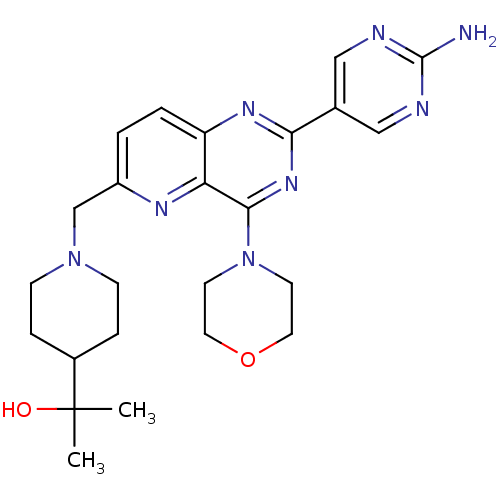
Affinity DataKi: 6.60E+3nMAssay Description:Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 minsMore data for this Ligand-Target Pair
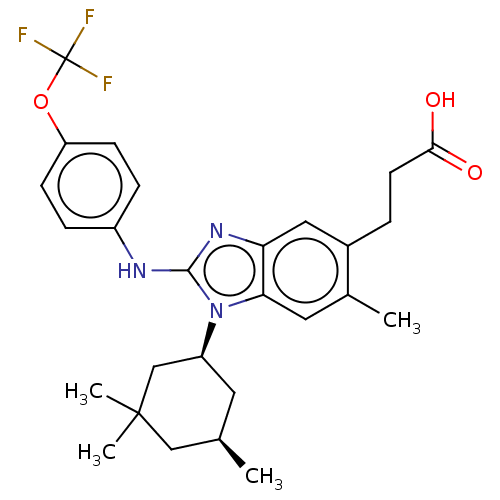
Affinity DataKi: 7.80E+4nMAssay Description:Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 minsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
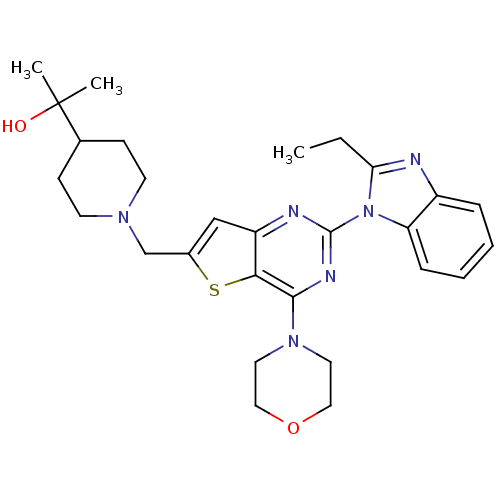
Affinity DataIC50: 0.480nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 0.670nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 5nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 5nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 9.80nMAssay Description:Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair

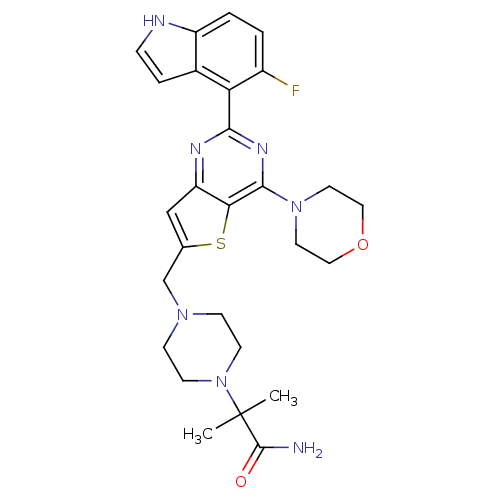
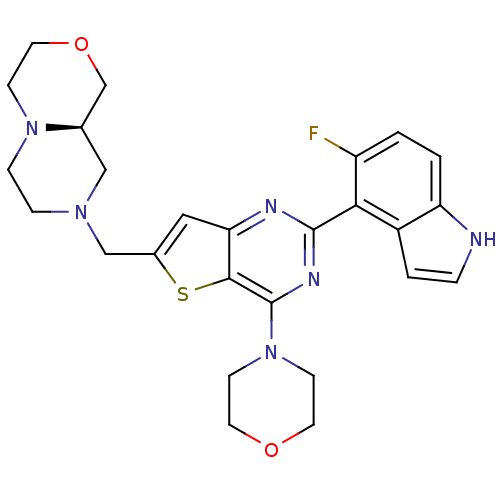
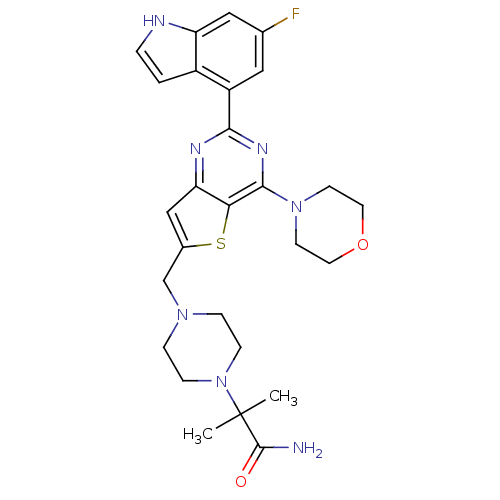
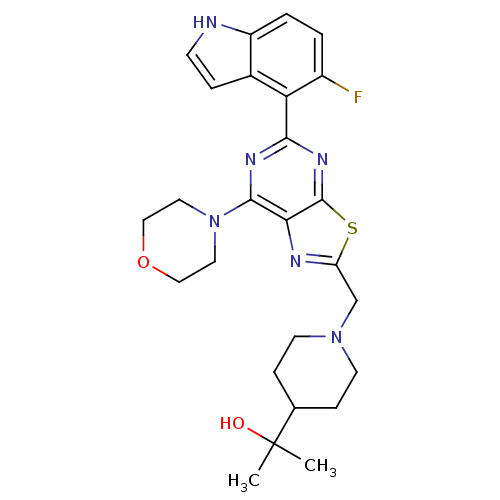
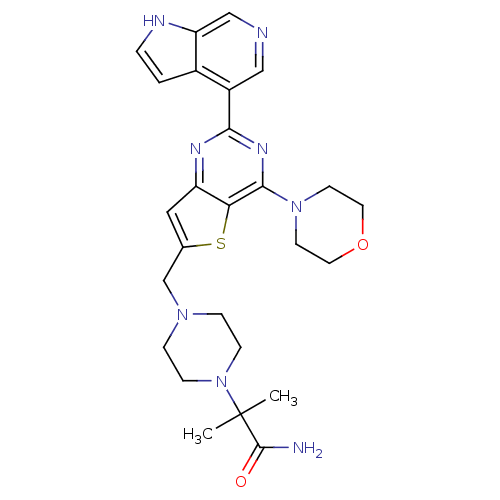
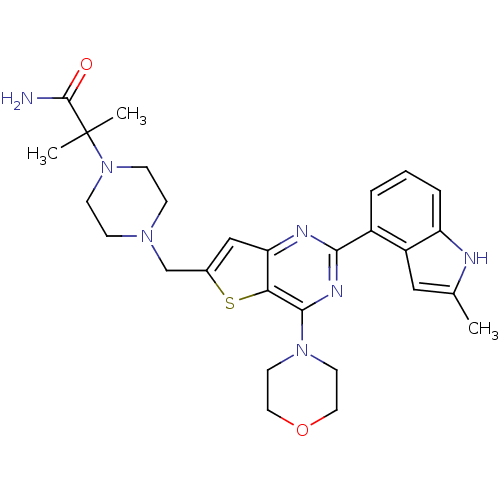
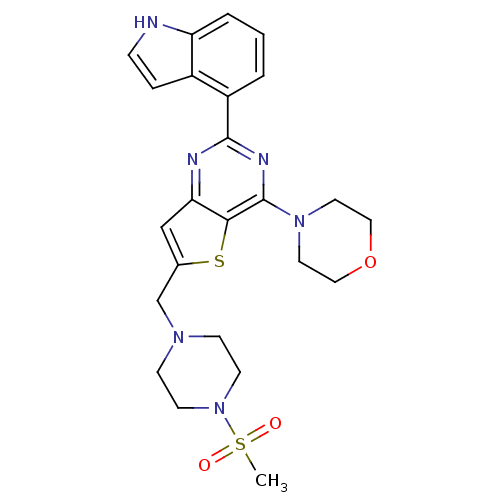
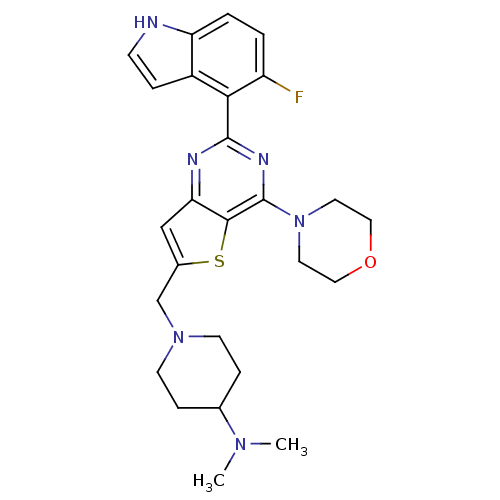
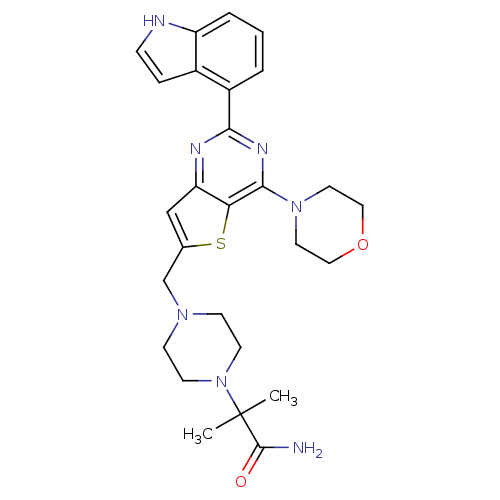
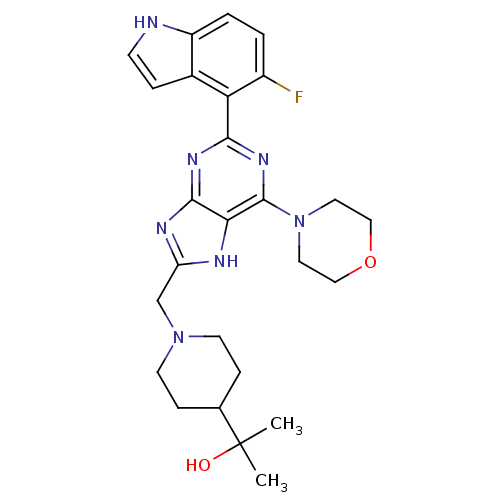
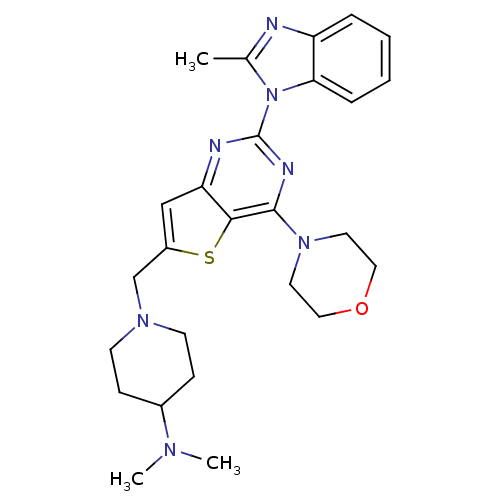
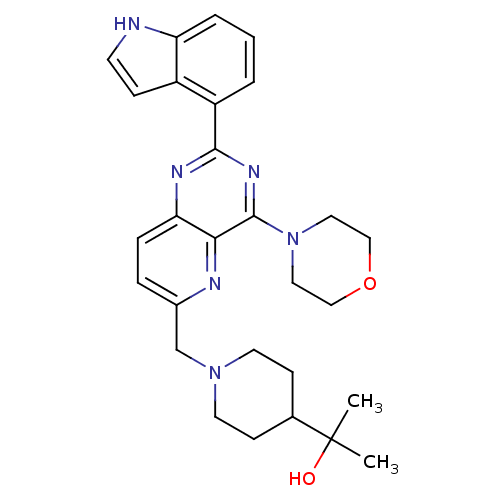
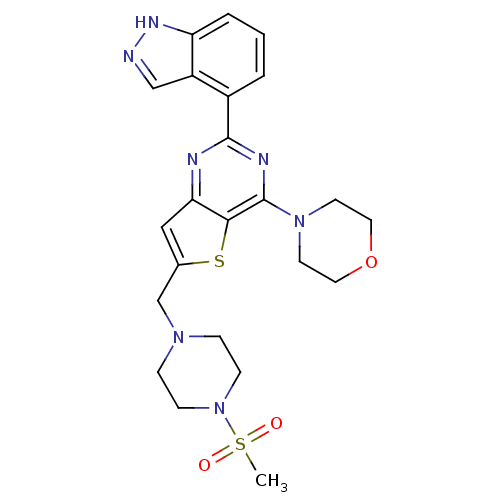
 3D Structure (crystal)
3D Structure (crystal)