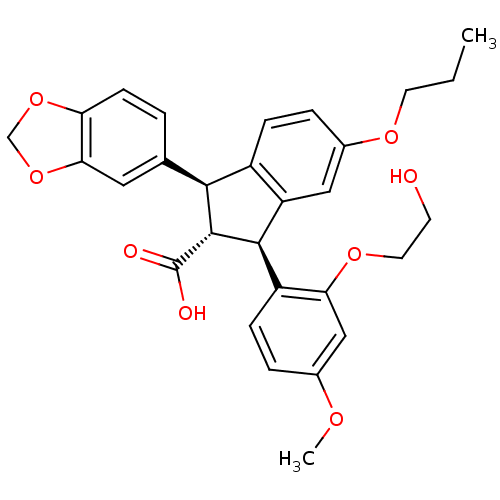
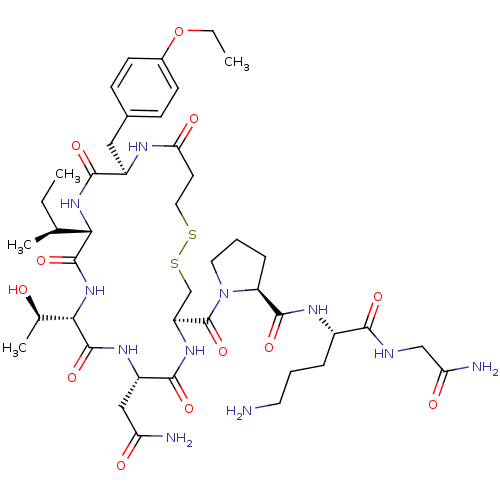
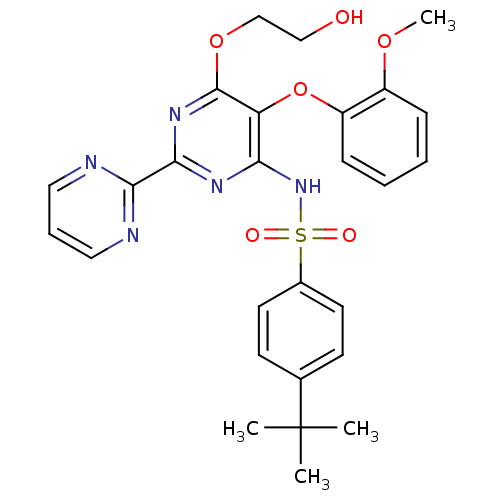
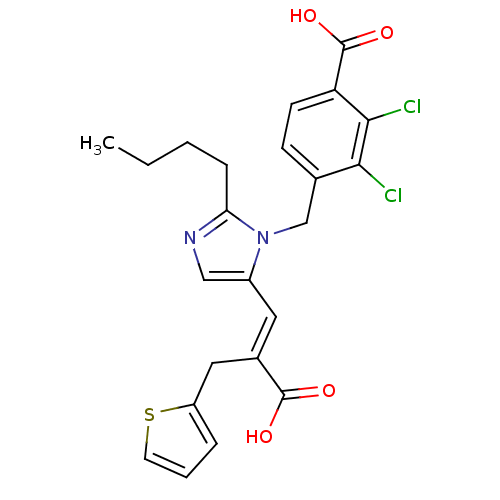
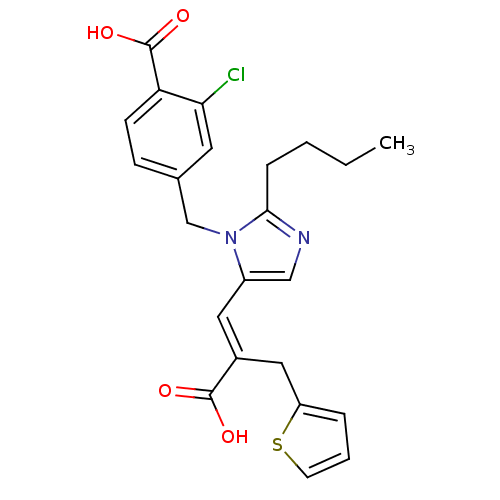
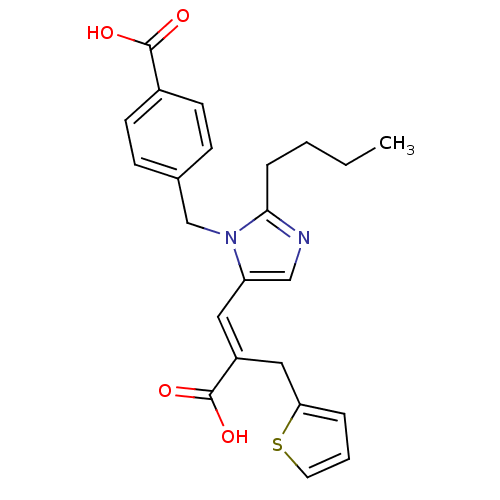
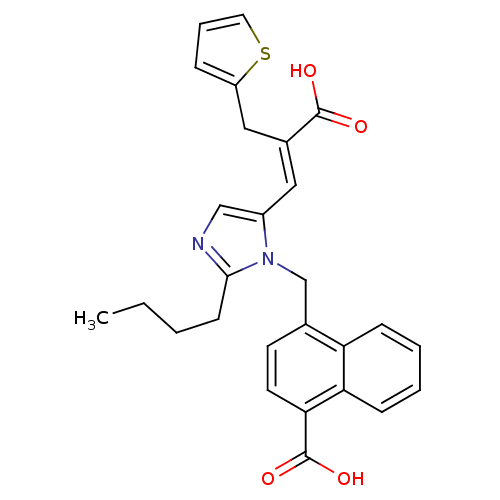
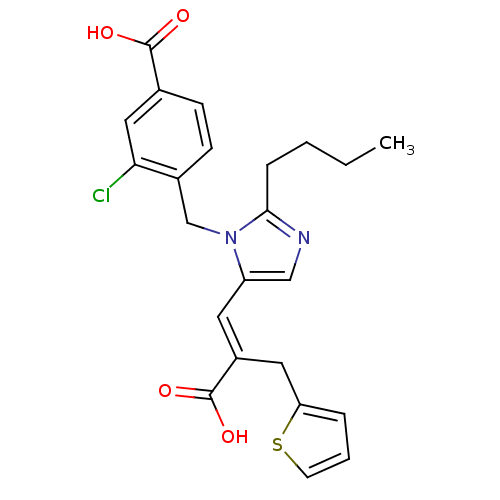
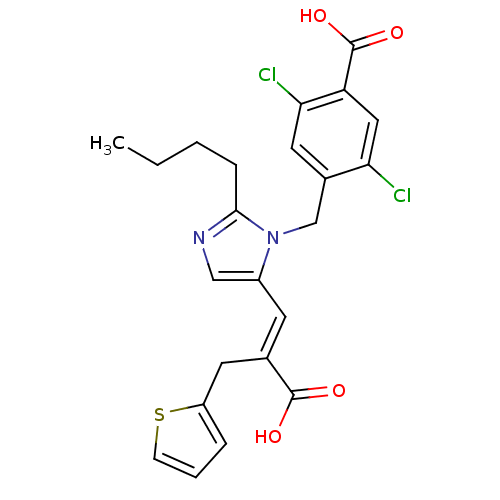
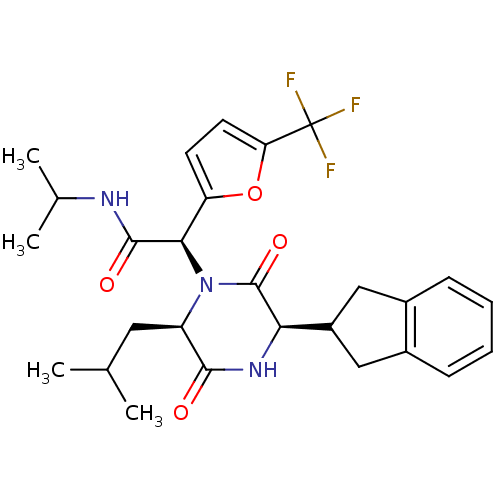
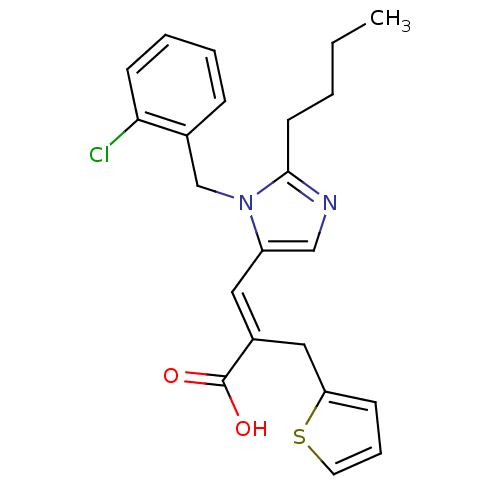
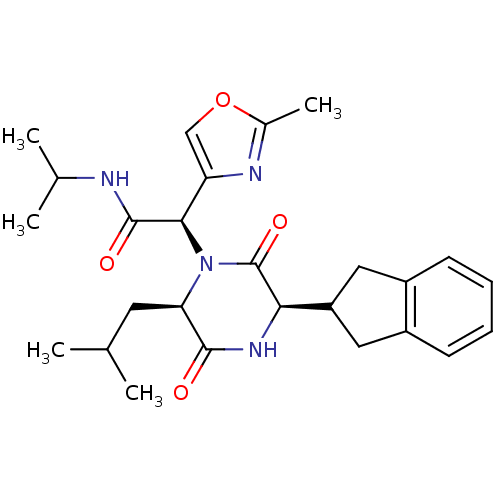
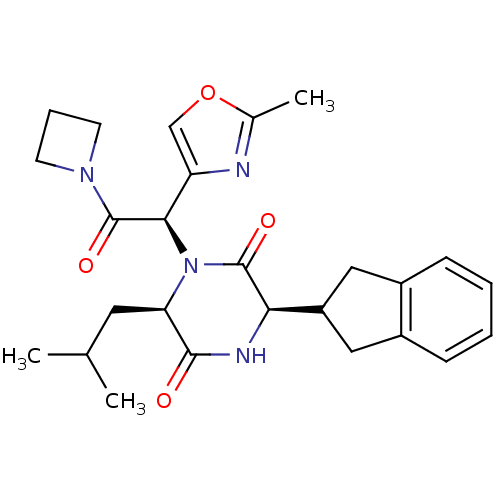
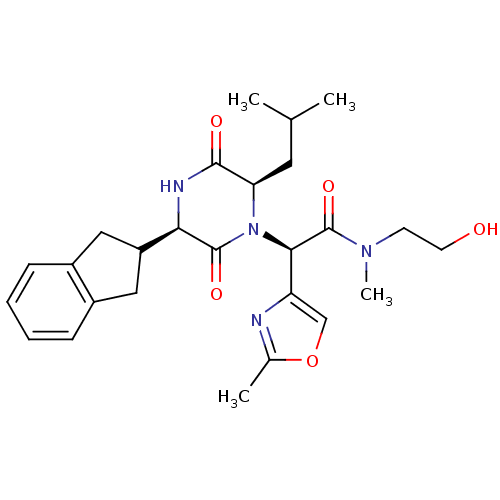
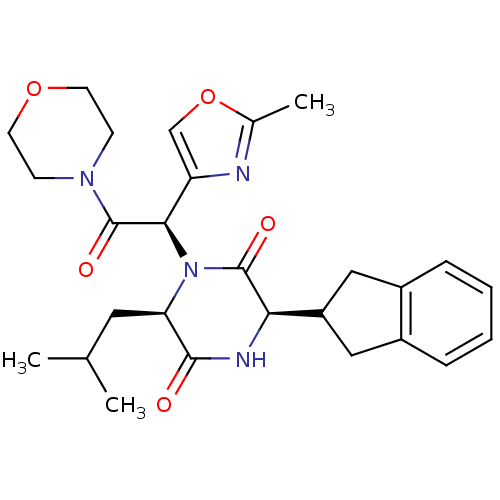
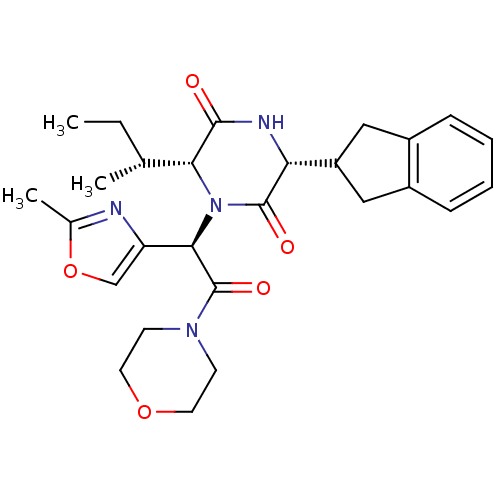
TargetEndothelin-1 receptor(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetEndothelin-1 receptor(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Affinity DataKi: 1nMAssay Description:Binding affinity to human oxytocin receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Affinity DataKi: 4.10nMAssay Description:Binding affinity to recombinant oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Binding affinity to human oxytocin receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Affinity DataKi: 32nMAssay Description:Binding affinity to recombinant oxytocin receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetEndothelin receptor type B(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetEndothelin receptor type B(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Affinity DataKi: 950nMAssay Description:Binding affinity to human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human vasopressin V1b receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.20E+4nMAssay Description:Binding affinity to human vasopressin V1a receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the absence of BSAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the absence of bovine serum albumin (BSA)More data for this Ligand-Target Pair
Affinity DataIC50: 7.30nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the presence of 0.25% bovine serum albumin (BSA)More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the presence of 0.25% BSAMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Tested for binding affinity to Angiotensin II receptor, type 1More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 4.53E+3nMAssay Description:Tested for binding affinity to Angiotensin II receptor, type 1More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair

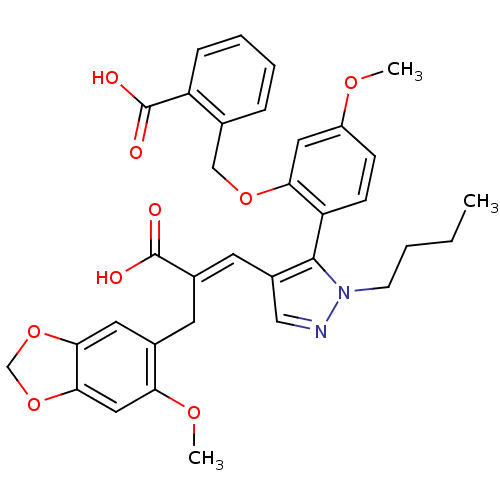
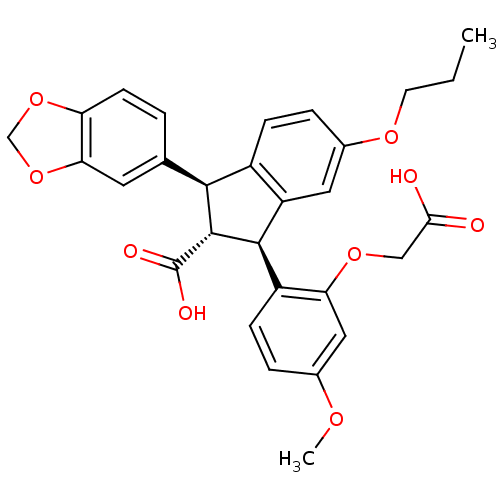
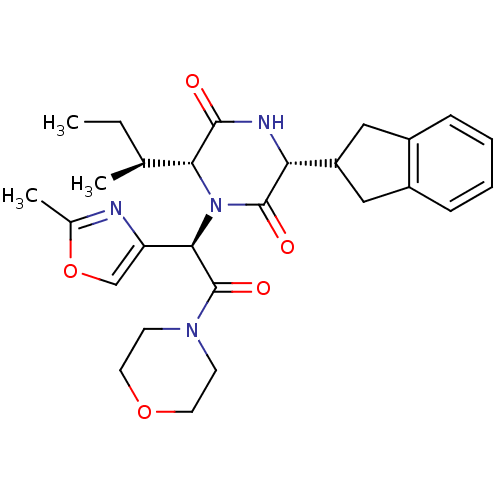
 3D Structure (crystal)
3D Structure (crystal)