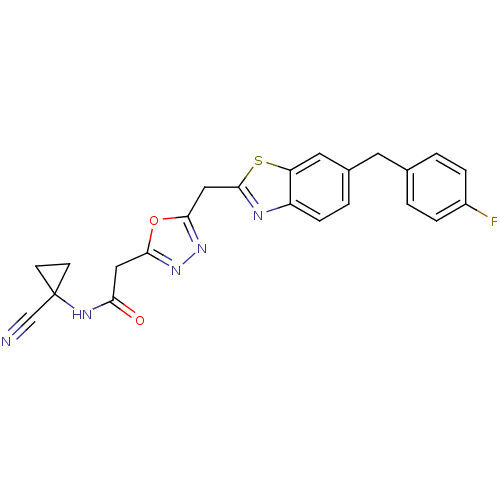
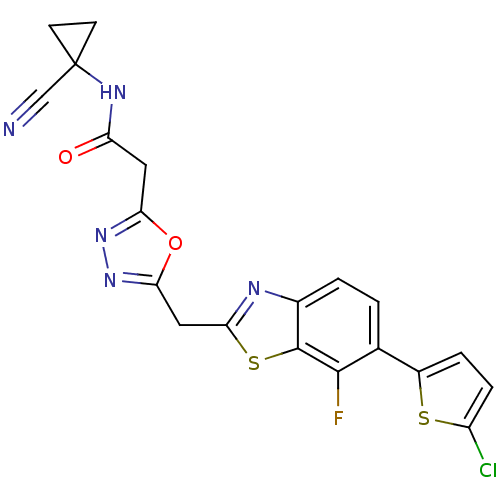
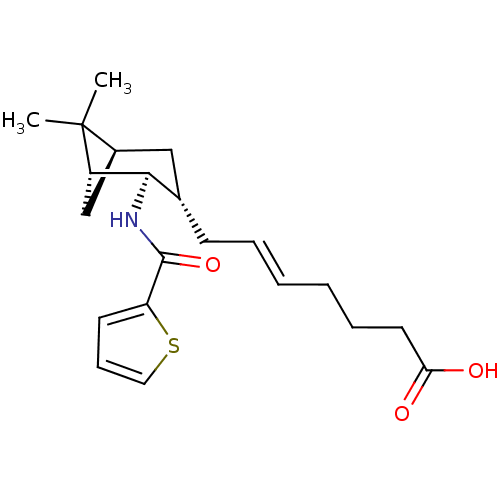
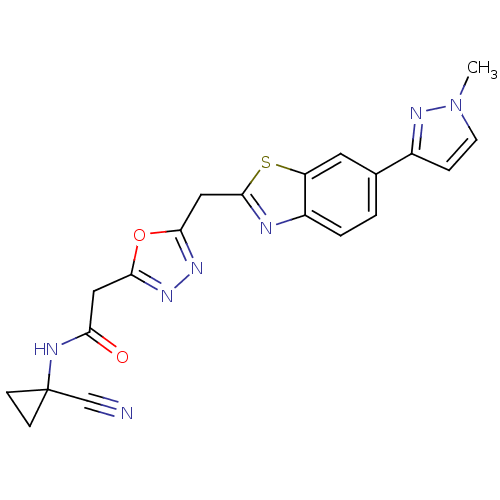
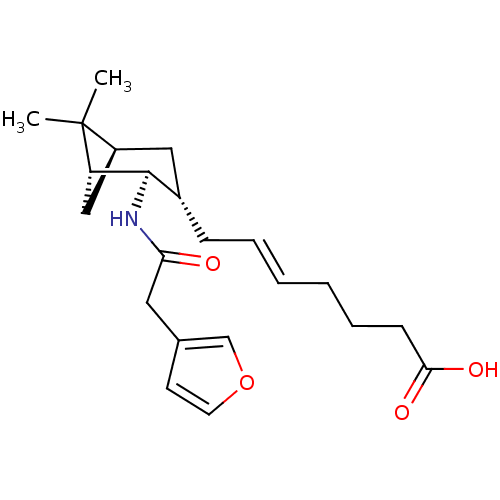
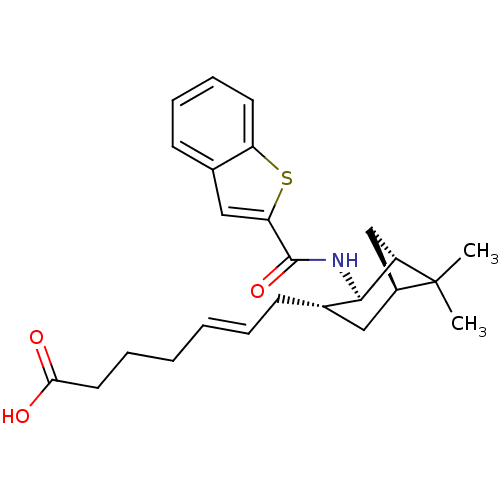
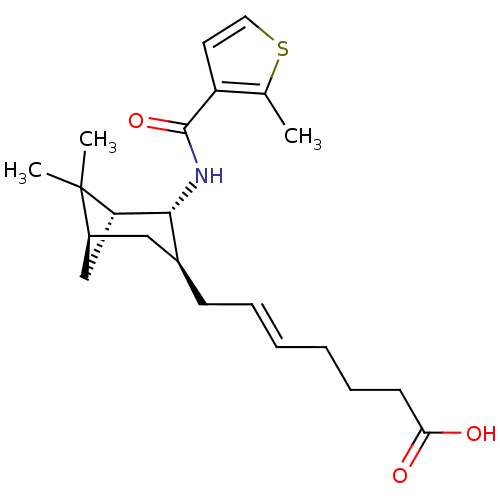
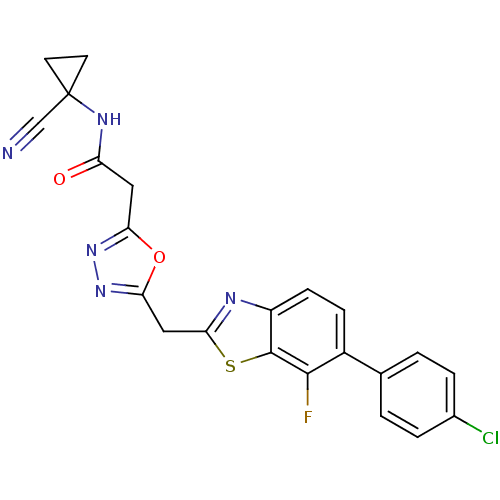
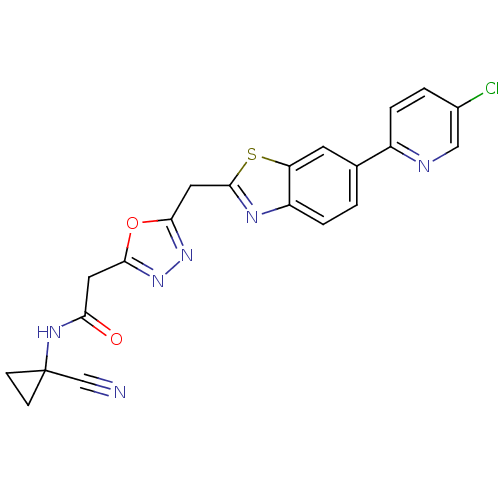
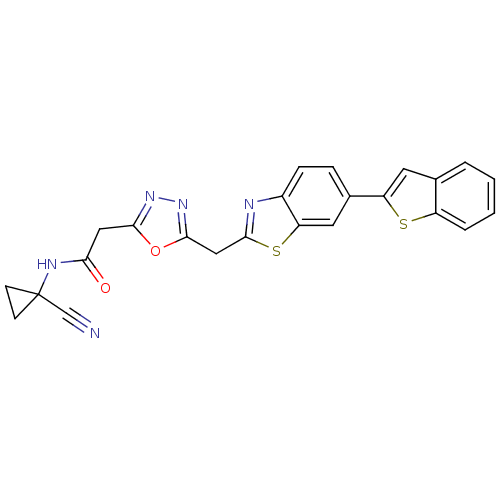
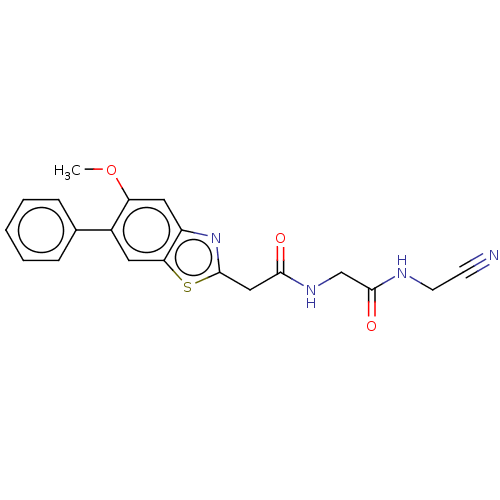
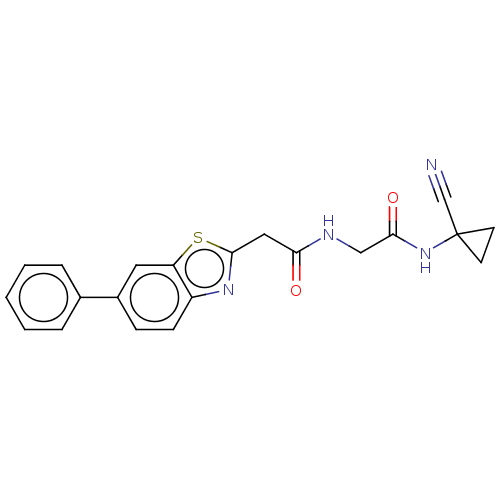
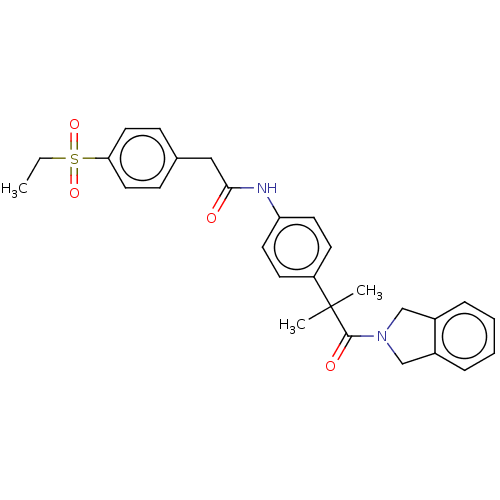
Affinity DataIC50: 0.420nMAssay Description:Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Transrepression of RORgammat in anti-CD3/CD28 stimulated human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Concentration required to inhibit the PGD-2 evoked cAMP formation in human plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMT: 2°CAssay Description:After the present compound dissolved in DMSO was added to become 0.5% DMSO to the reaction buffer consisting of 20 mM tris hydrochloric acid (pH7.4),...More data for this Ligand-Target Pair
Affinity DataIC50: <30nMAssay Description:Displacement of [3H]-Digoxin from recombinant human GST-fused RORgammat LBD after 1 to 4 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: <30nMAssay Description:Displacement of [3H]-Digoxin from recombinant human GST-fused RORgammat LBD after 1 to 4 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: <30nMAssay Description:Displacement of [3H]-Digoxin from recombinant human GST-fused RORgammat LBD after 1 to 4 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: <30nMAssay Description:Displacement of [3H]-Digoxin from recombinant human GST-fused RORgammat LBD after 1 to 4 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: <30nMAssay Description:Displacement of [3H]-Digoxin from recombinant human GST-fused RORgammat LBD after 1 to 4 hrs by scintillation counting methodMore data for this Ligand-Target Pair

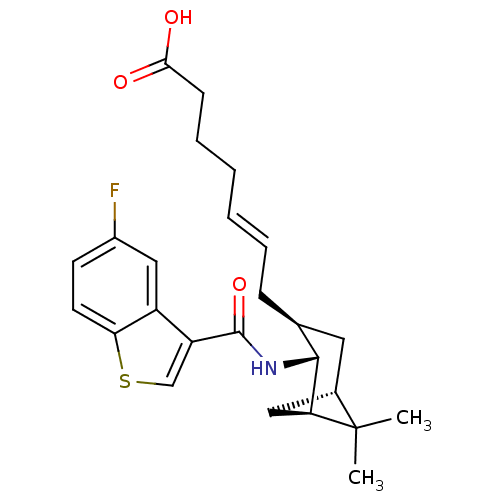
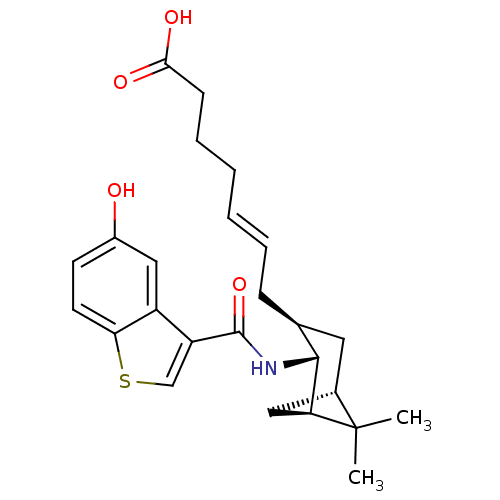
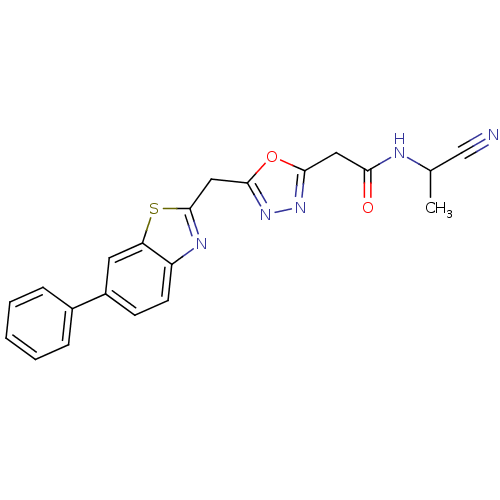
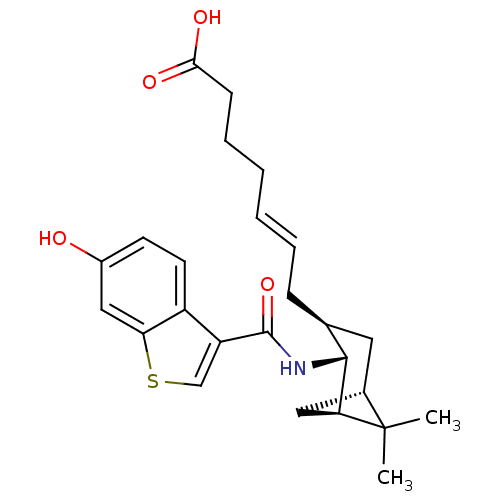
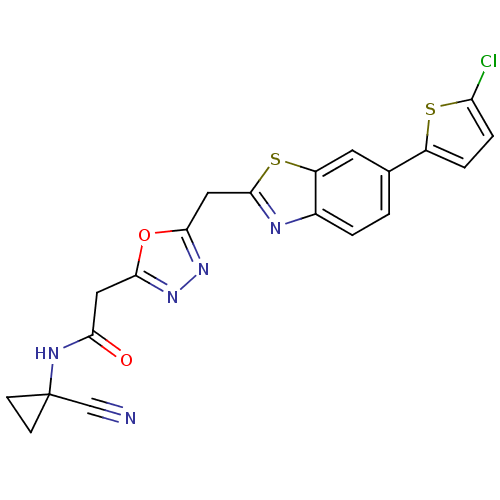
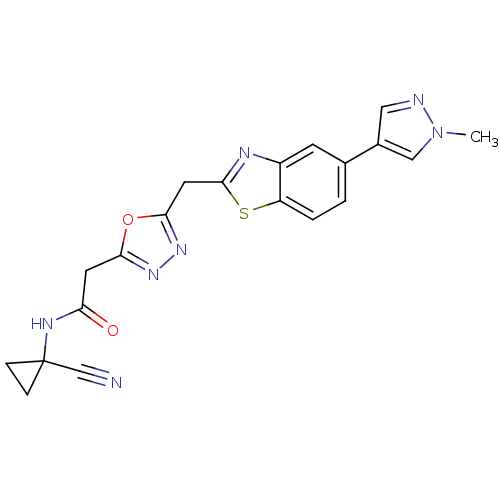
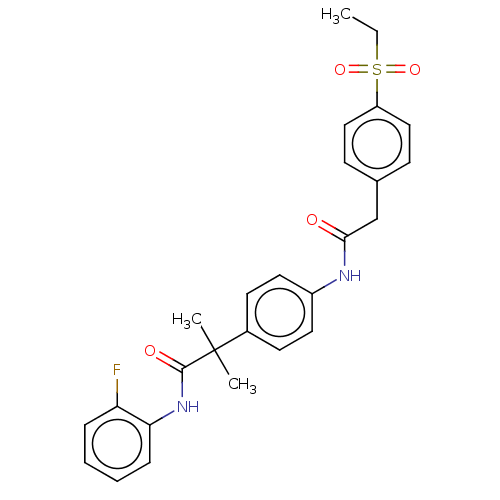
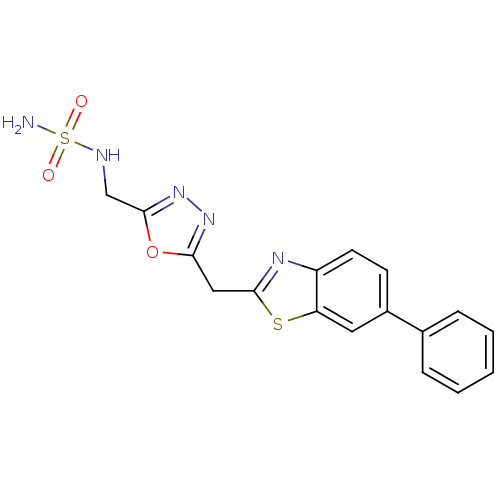
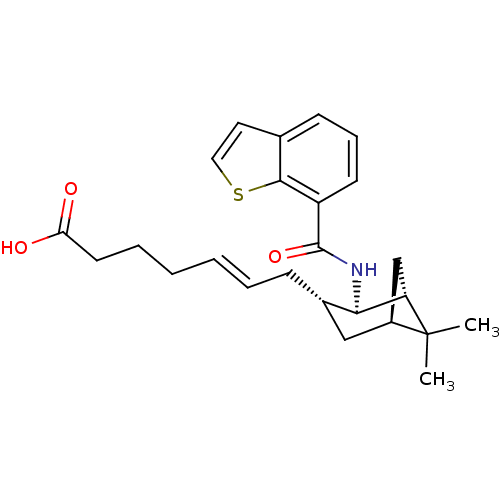
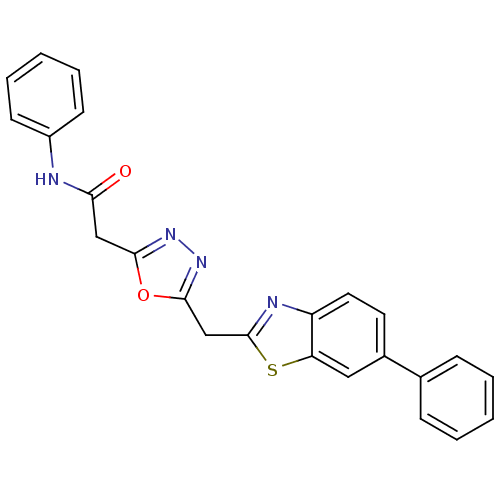
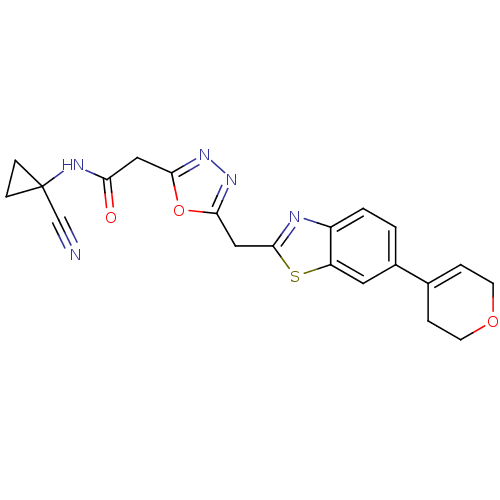
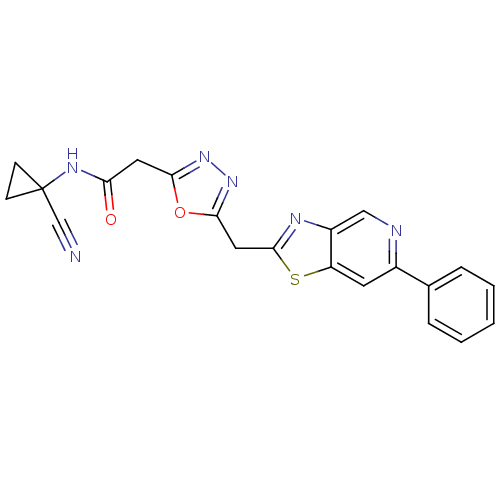
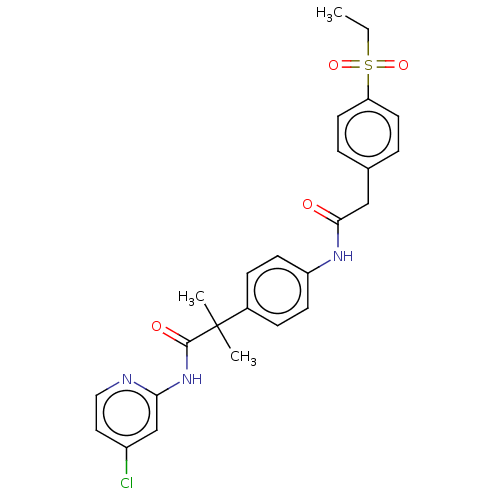
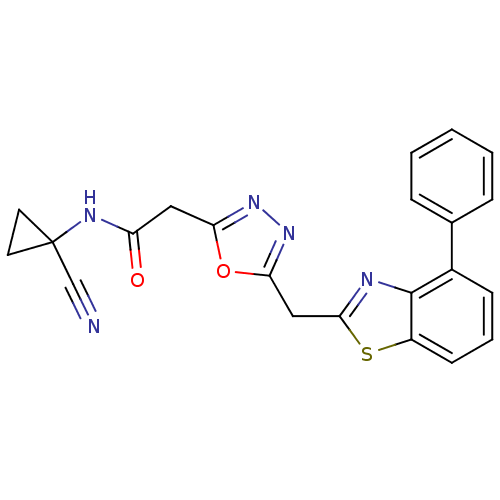
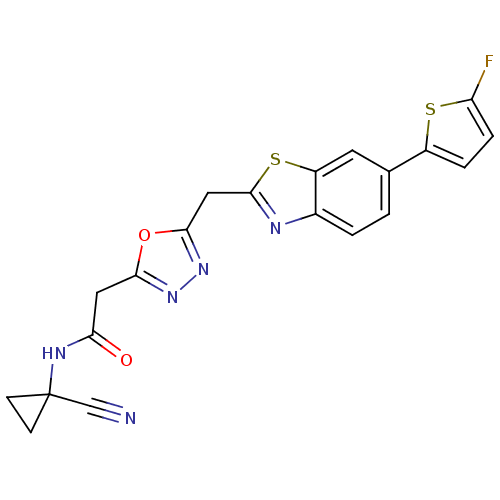
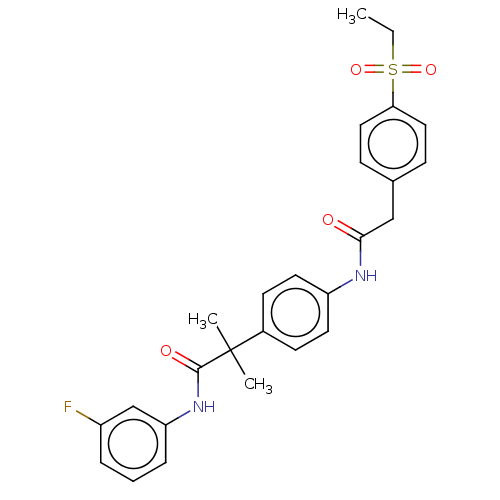

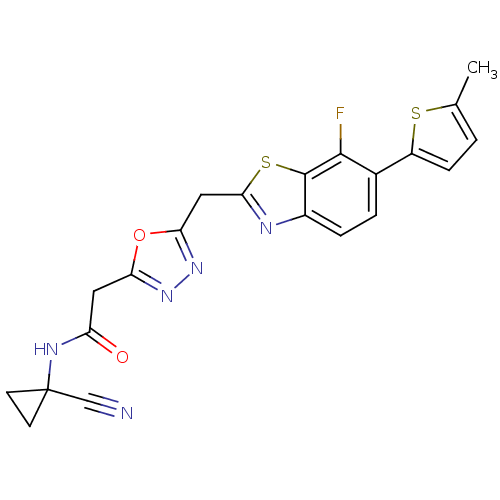
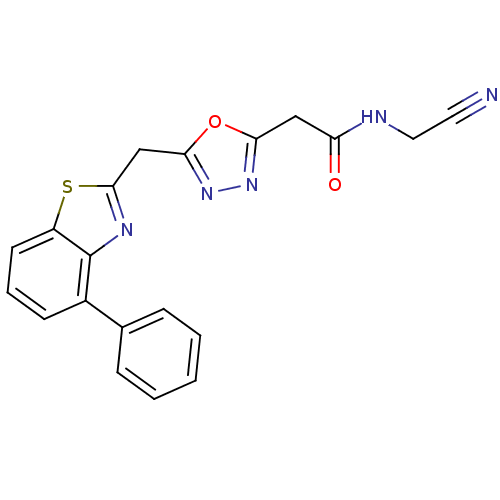
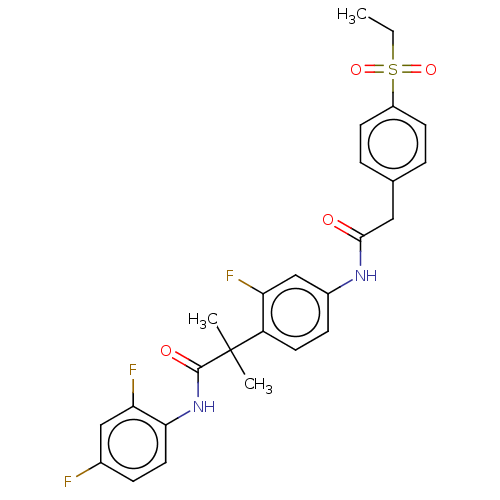

 3D Structure (crystal)
3D Structure (crystal)