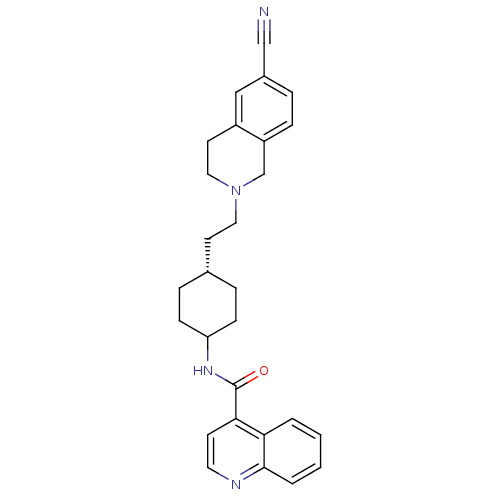
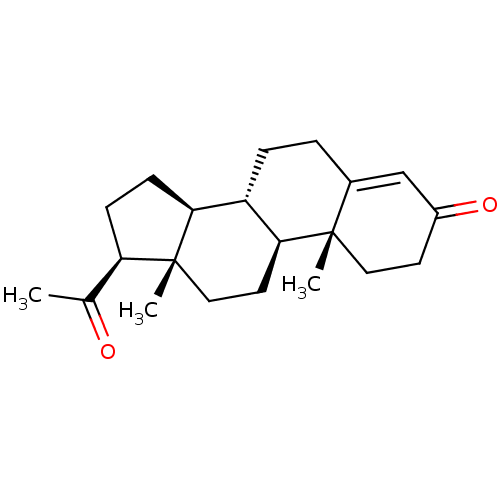
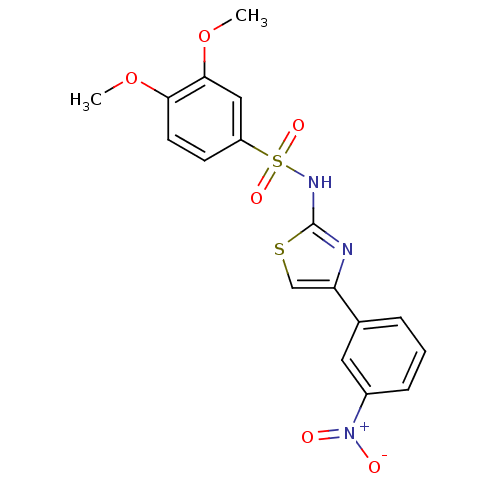
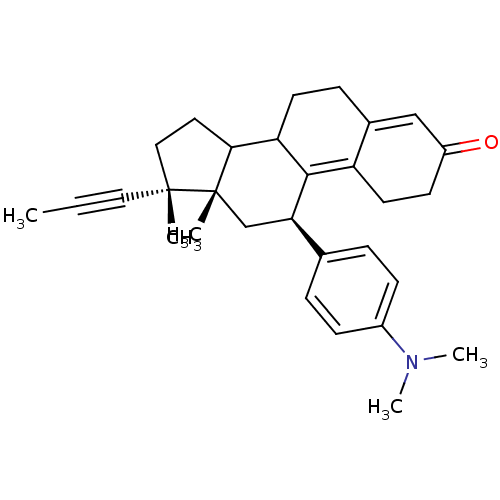
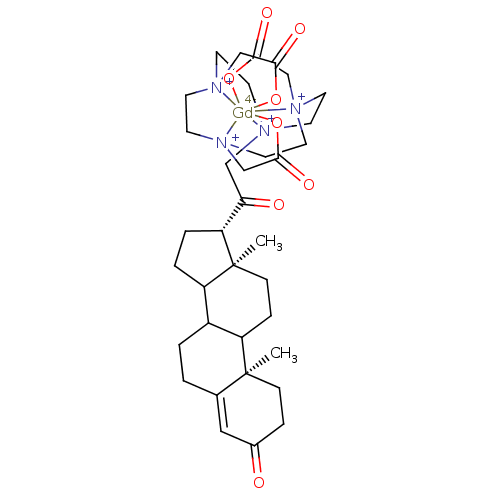
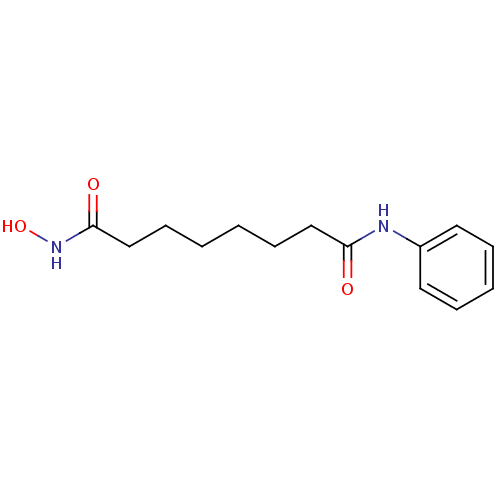
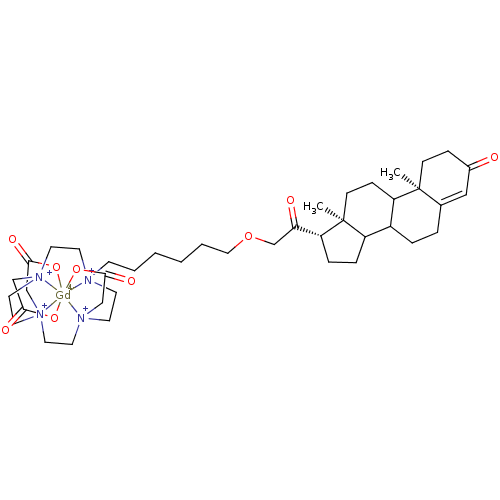
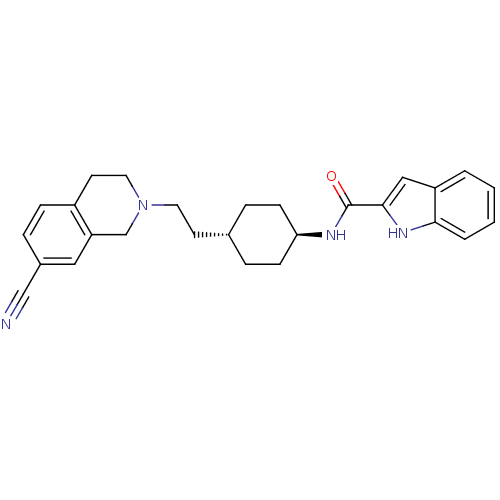
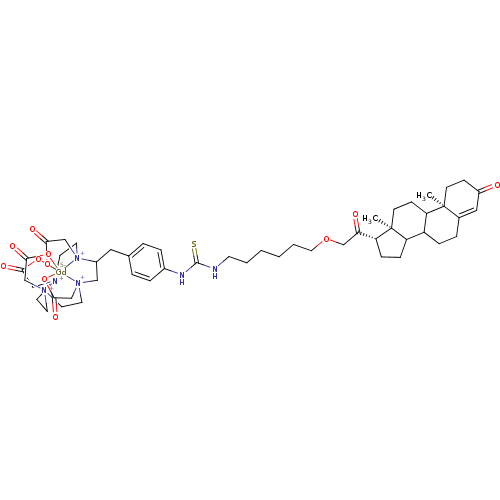
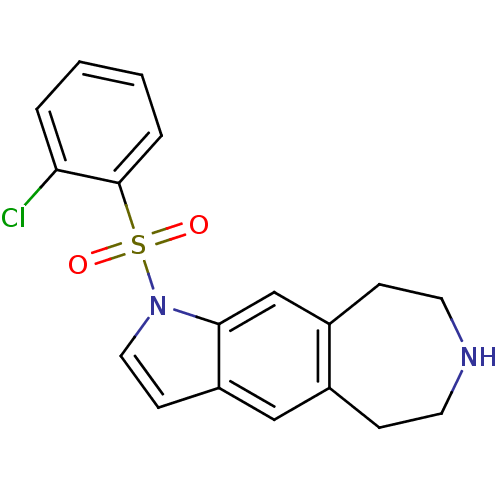
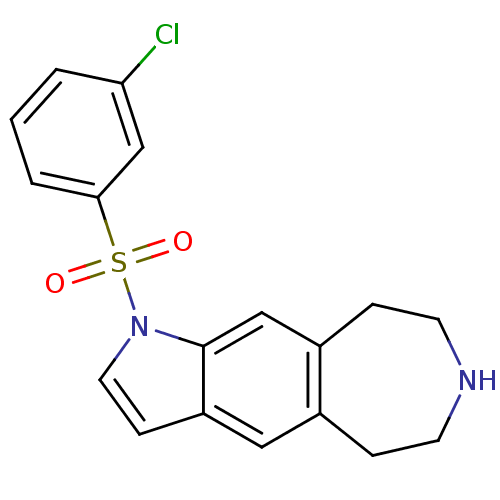
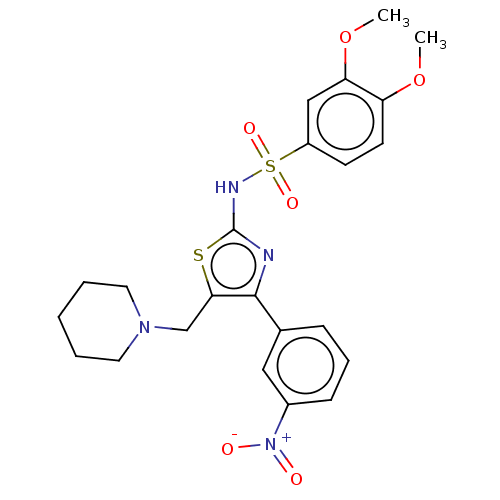
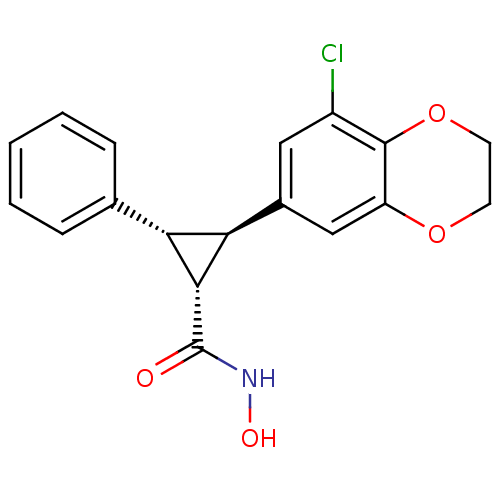
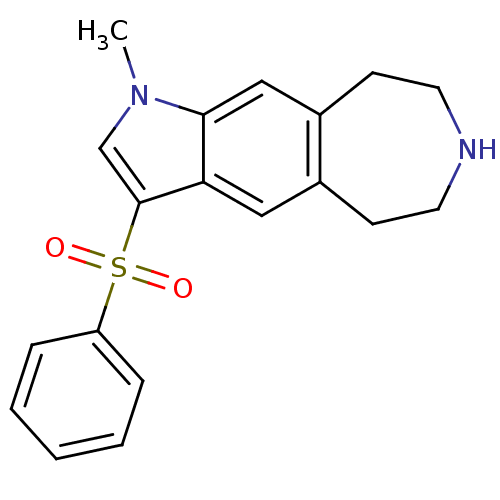
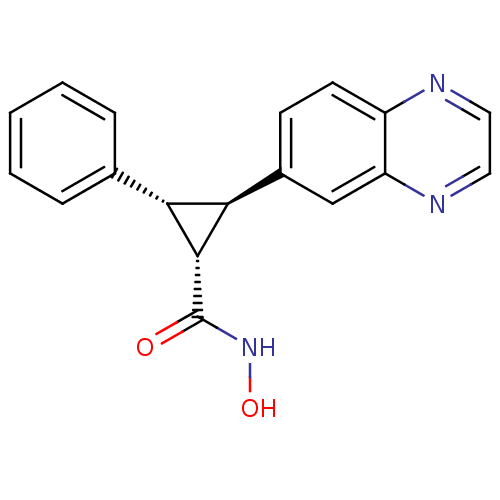
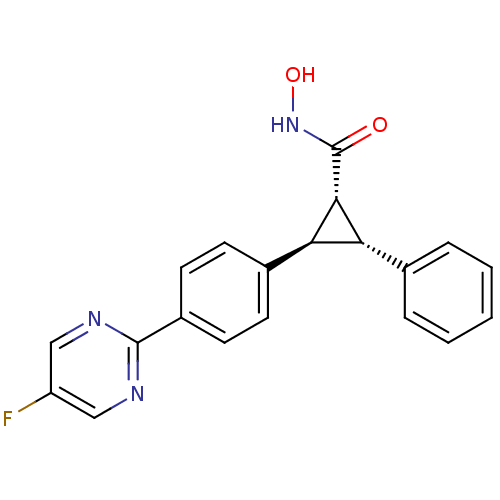
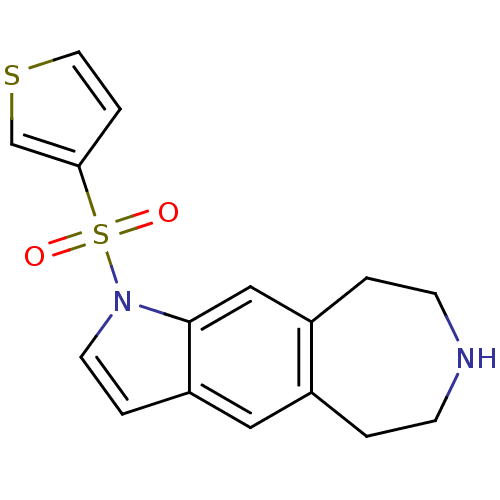
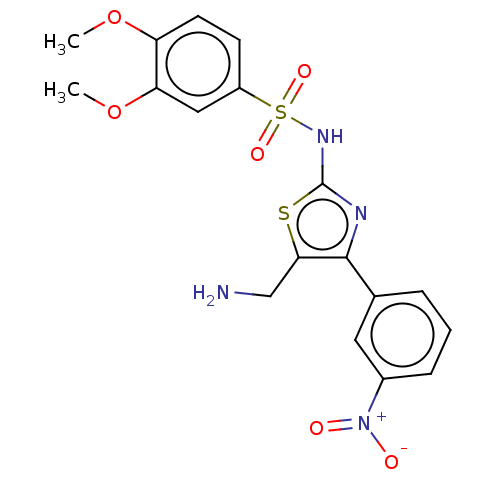
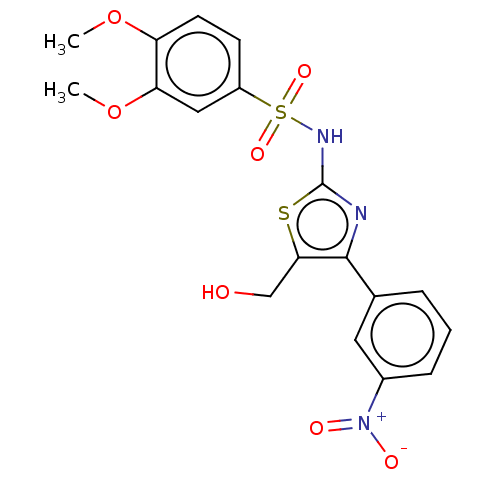
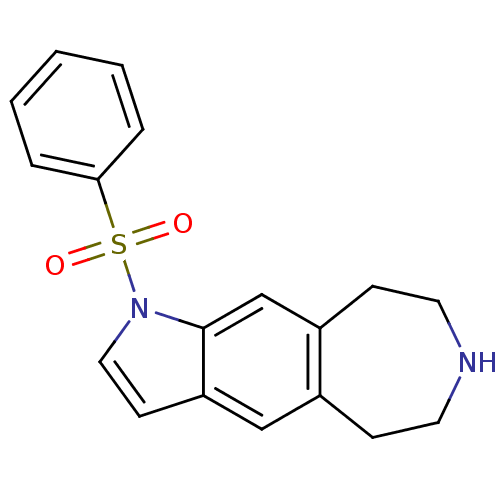
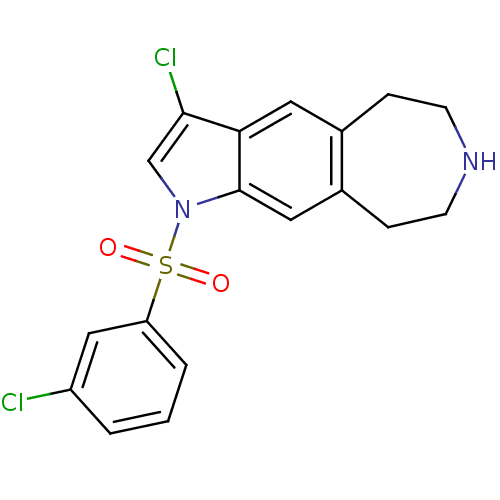
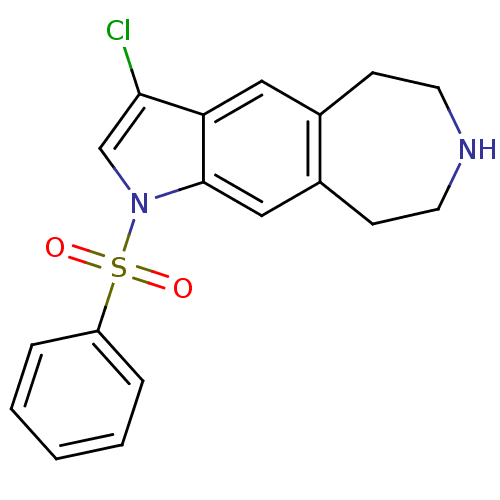
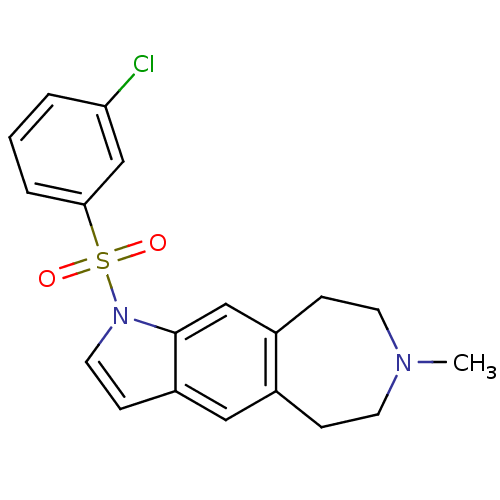
TargetD(3) dopamine receptor(Rattus norvegicus (Rat))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetD(3) dopamine receptor(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 4(GUINEA PIG)
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1F(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1B(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1E(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Smithkline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Affinity DataIC50: 1.60nMT: 2°CAssay Description:Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMT: 2°CAssay Description:Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of C57BL/6J mouse KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS a...More data for this Ligand-Target Pair
Affinity DataIC50: 96nMT: 2°CAssay Description:Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. More data for this Ligand-Target Pair
TargetKynurenine 3-monooxygenase(Homo sapiens (Human))
Chdi Management/Chdi Foundation
Curated by ChEMBL
Chdi Management/Chdi Foundation
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of human KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant full length HDAC3-NCoR2 using Lys_Ac_AMC as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of human recombinant full length HDAC3-NCoR2 using Lys_Ac_AMC as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 460nMT: 2°CAssay Description:Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataIC50: 860nMT: 2°CAssay Description:Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using PPR substrateMore data for this Ligand-Target Pair
TargetKynurenine 3-monooxygenase(Homo sapiens (Human))
Chdi Management/Chdi Foundation
Curated by ChEMBL
Chdi Management/Chdi Foundation
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of KMO (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human recombinant full length HDAC3-NCoR2 using Lys_Ac_AMC as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using PPR substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy...More data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+3nMAssay Description:Inhibition of human recombinant full length HDAC3-NCoR2 using Lys_Ac_AMC as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of human recombinant full length HDAC3-NCoR2 using Lys_Ac_AMC as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.70E+3nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy...More data for this Ligand-Target Pair
TargetKynurenine 3-monooxygenase(Homo sapiens (Human))
Chdi Management/Chdi Foundation
Curated by ChEMBL
Chdi Management/Chdi Foundation
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of C57BL/6J mouse KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human recombinant full length HDAC3-NCoR2 using Lys_Ac_AMC as substrate after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using PPR substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrateMore data for this Ligand-Target Pair