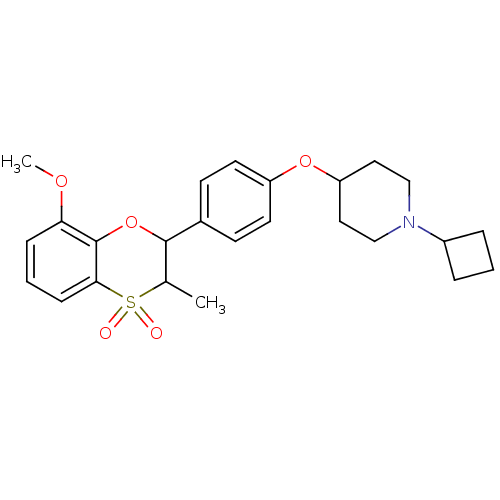
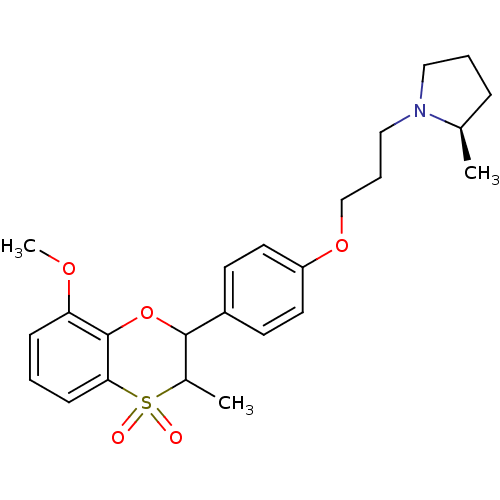
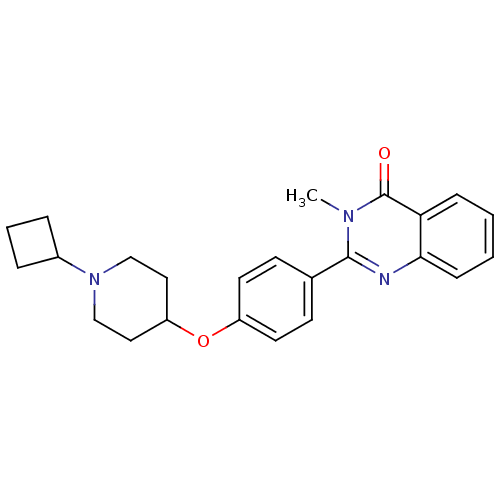
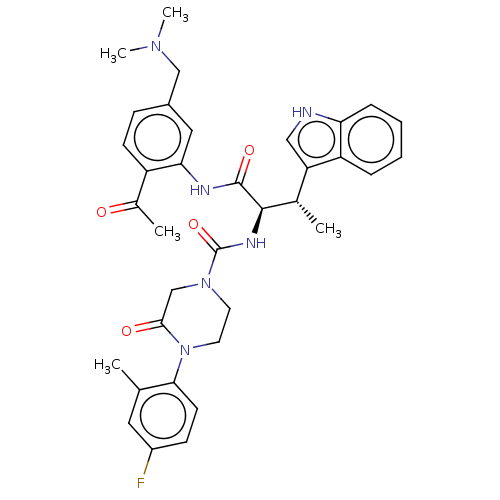
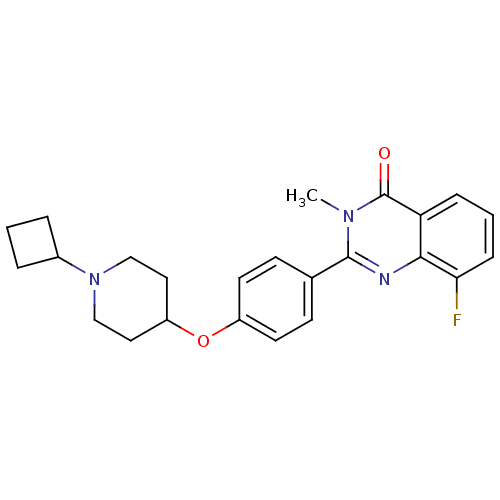
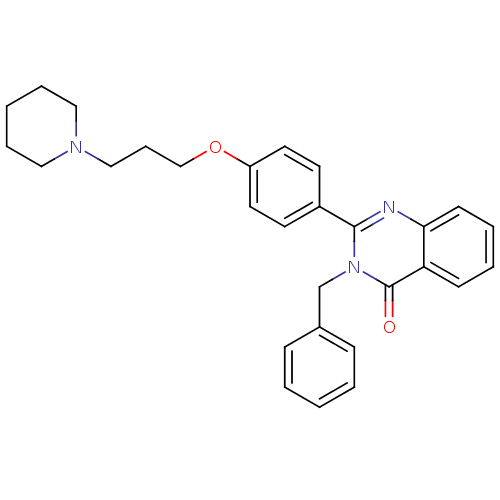
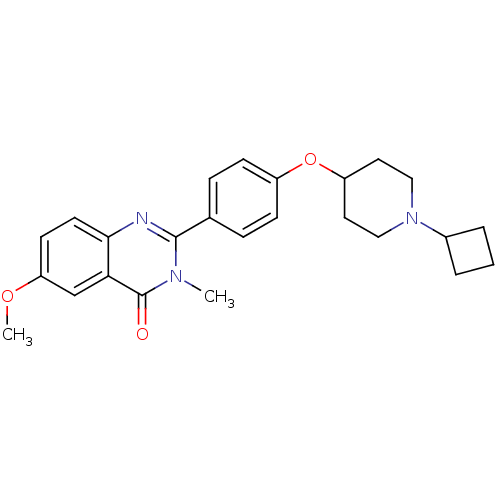
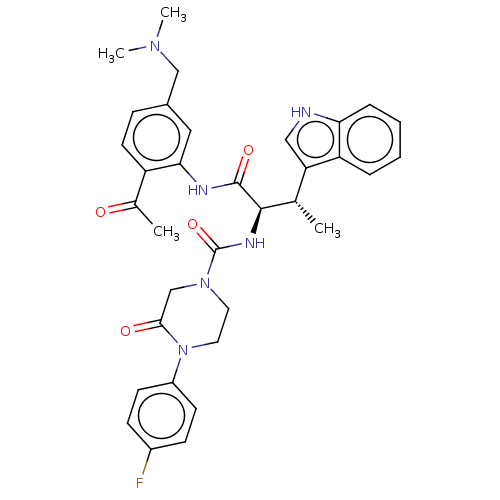
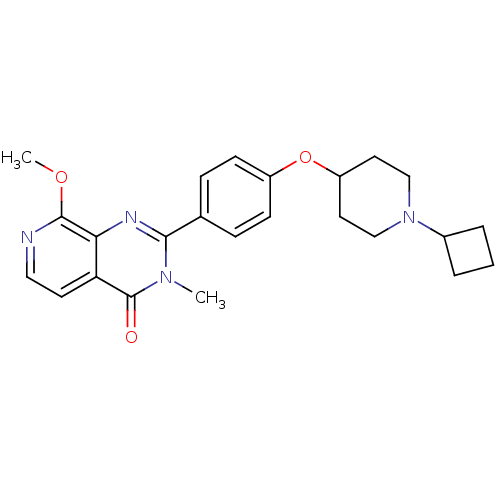
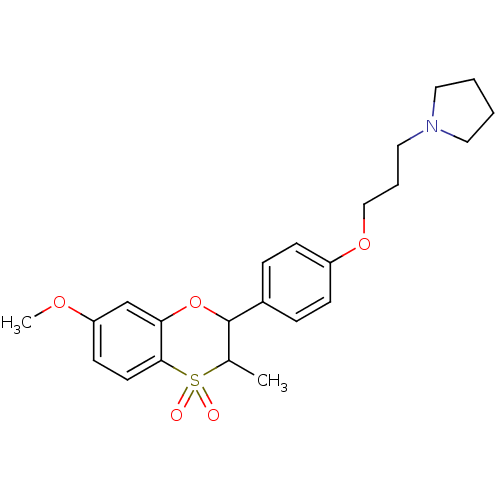
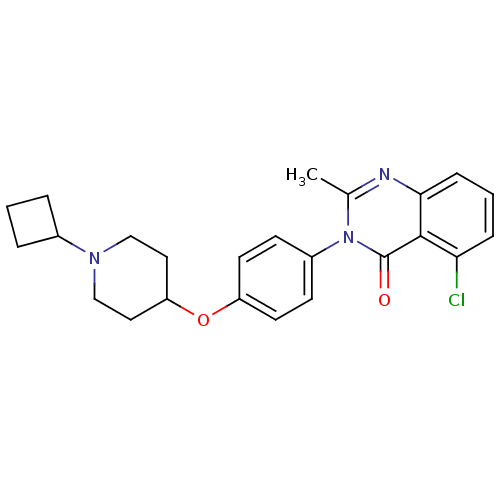
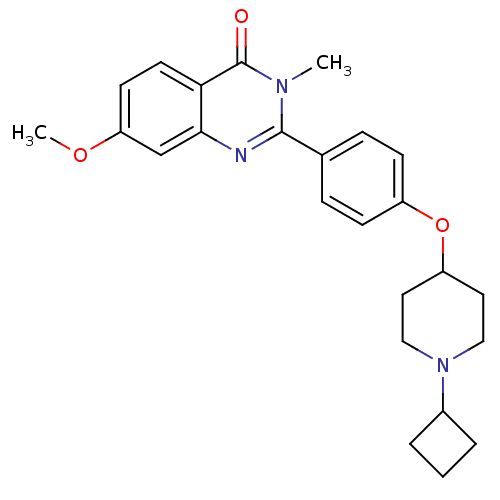
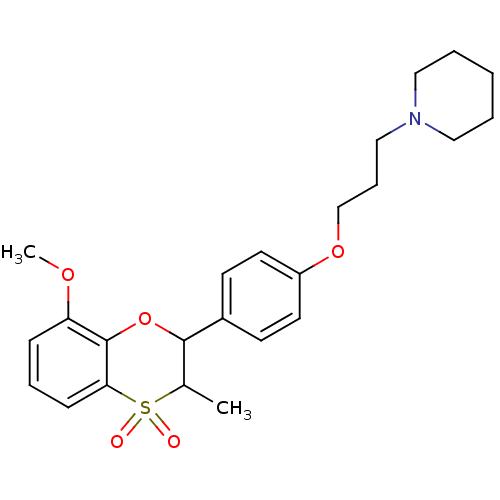
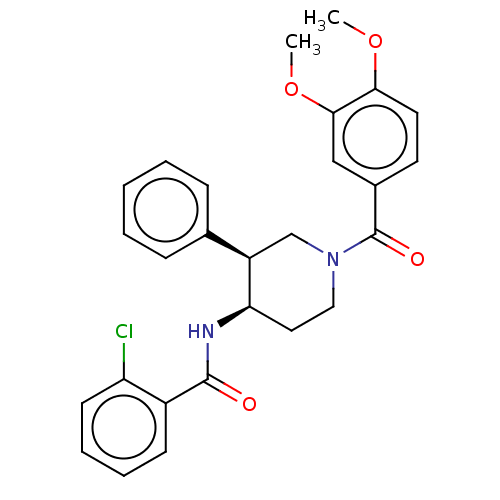
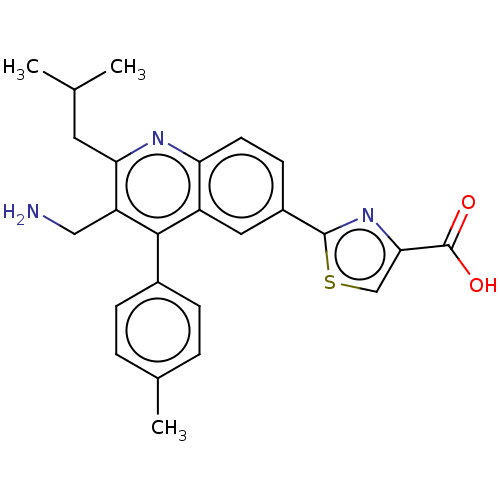
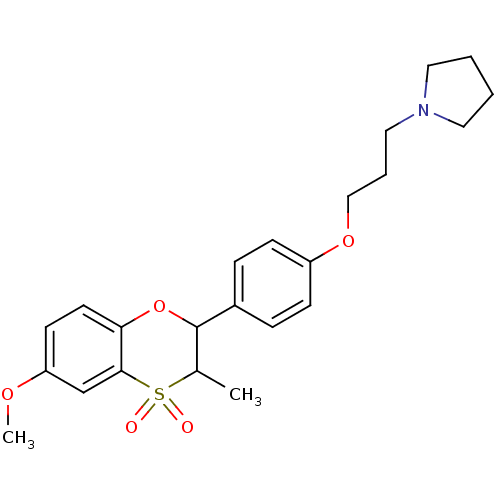
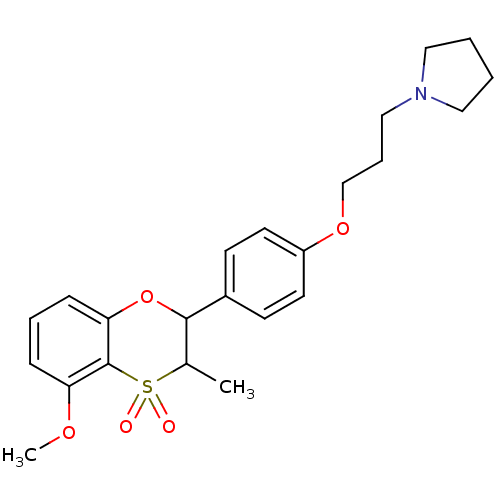
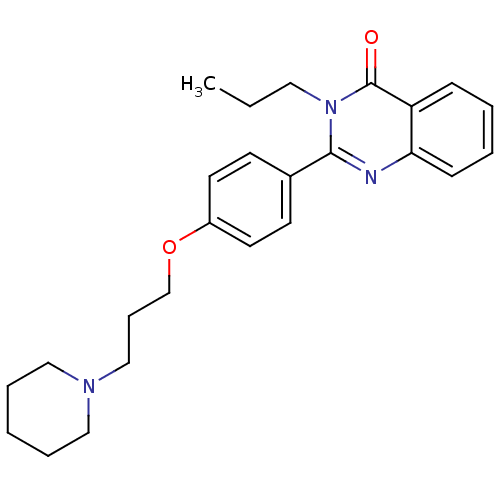
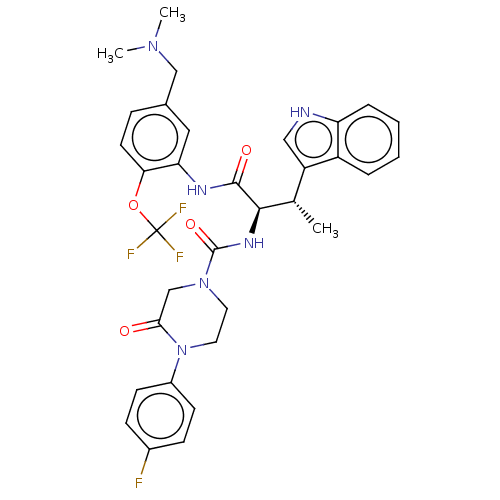
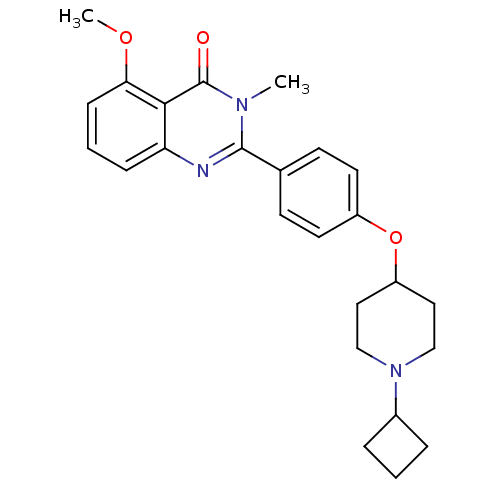
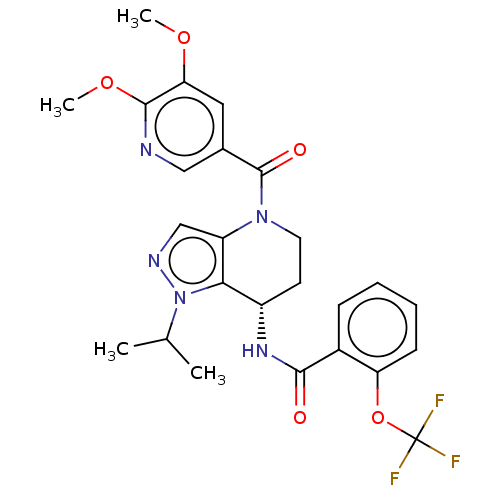
Affinity DataKi: 1nMAssay Description:Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
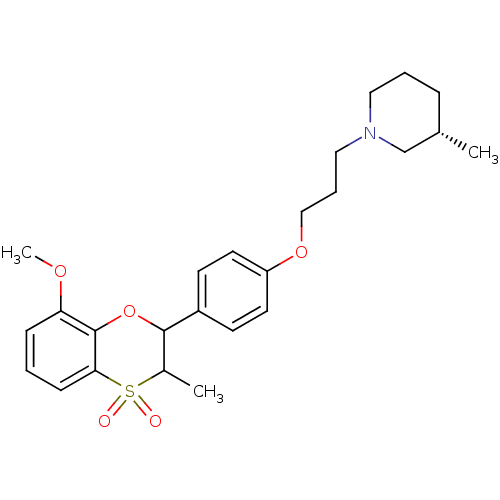
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
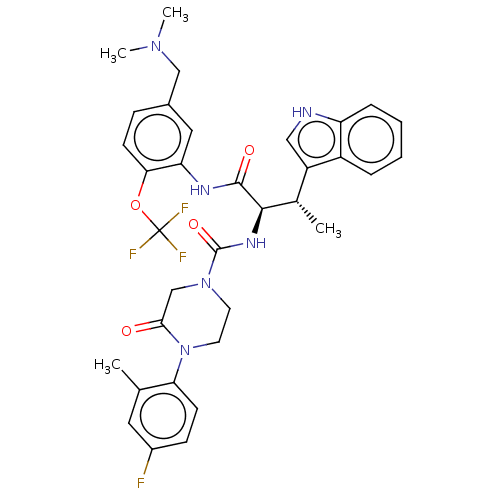
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
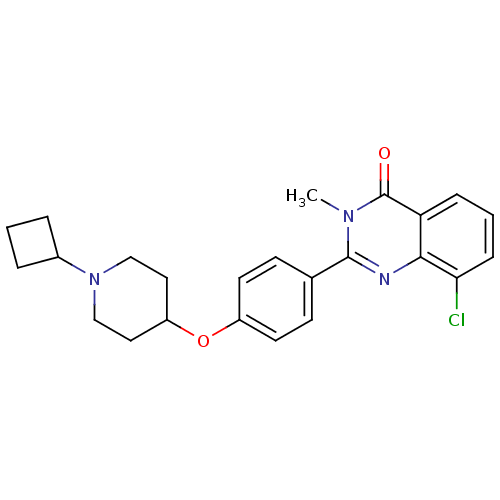
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine receptor H3(Macaca mulatta (Rhesus macaque))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8.40nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT...More data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR2 expressed in CHO dhfr cell membranes after 60 mins by TopCount scintillation counting ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair

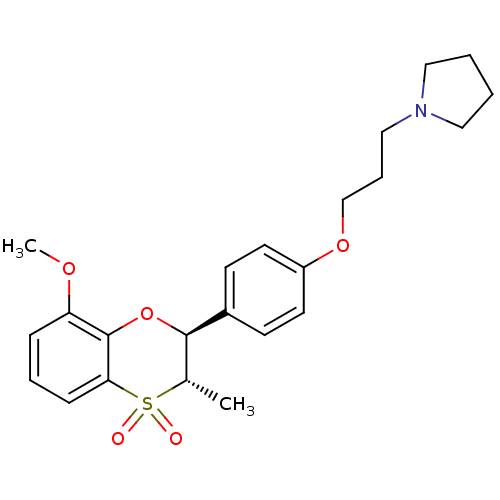
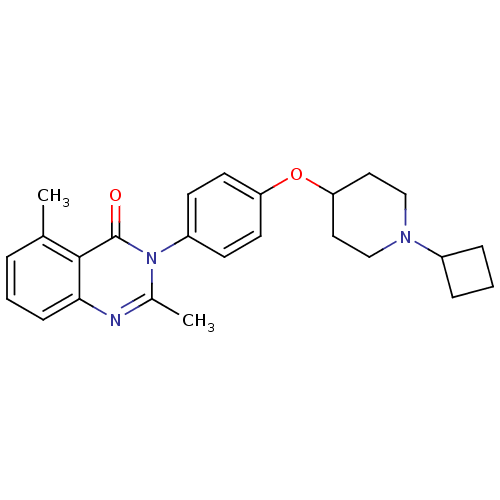
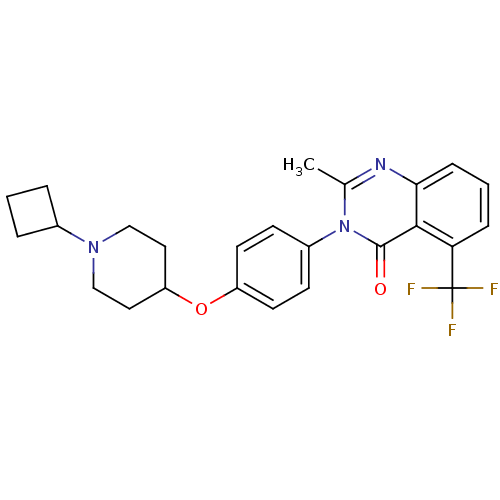
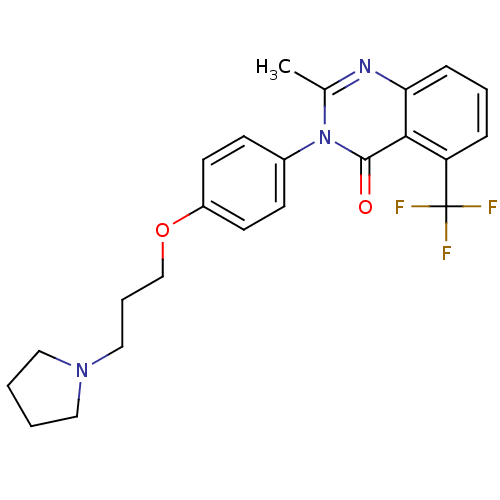
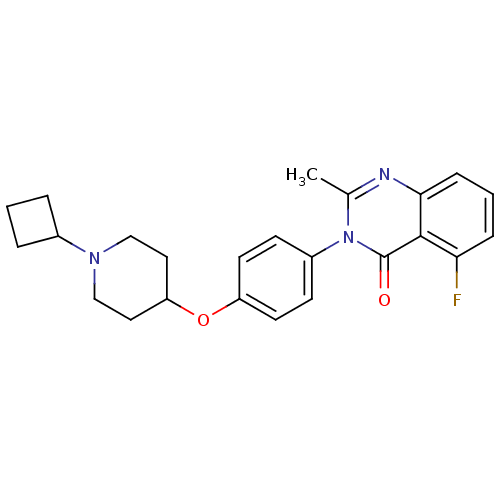
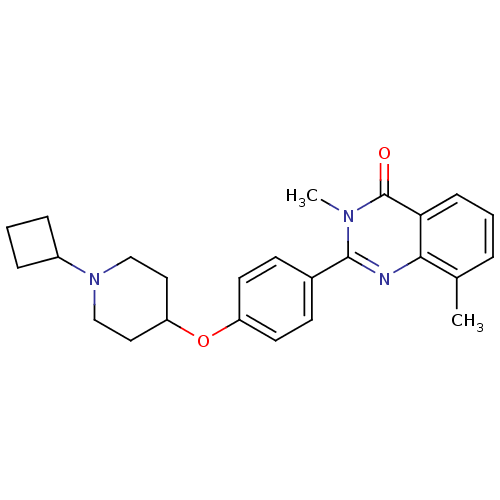
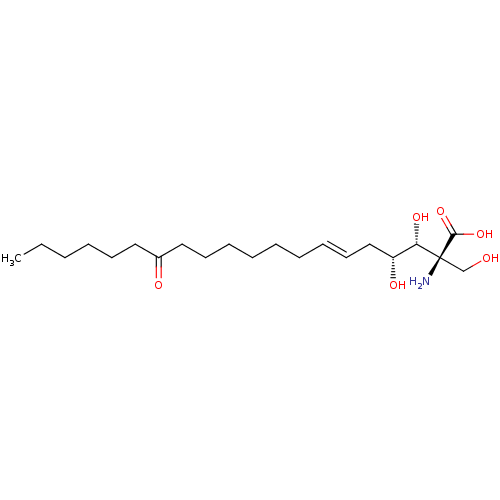
 3D Structure (crystal)
3D Structure (crystal)