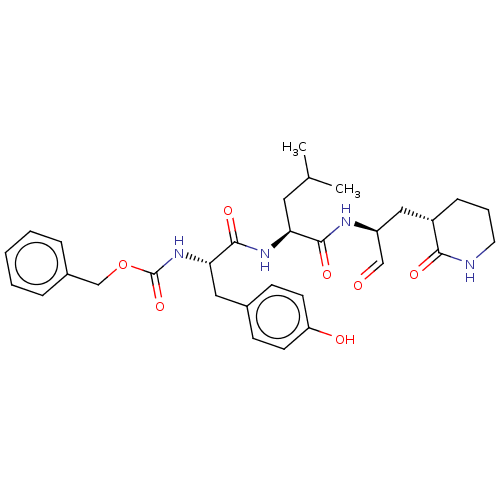
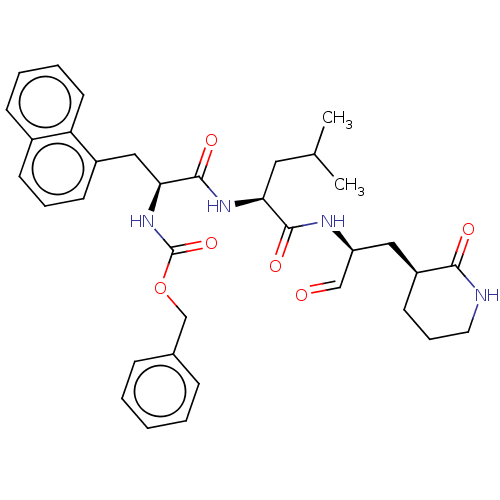
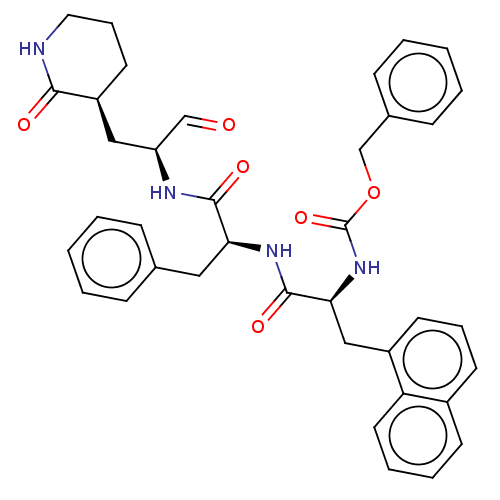
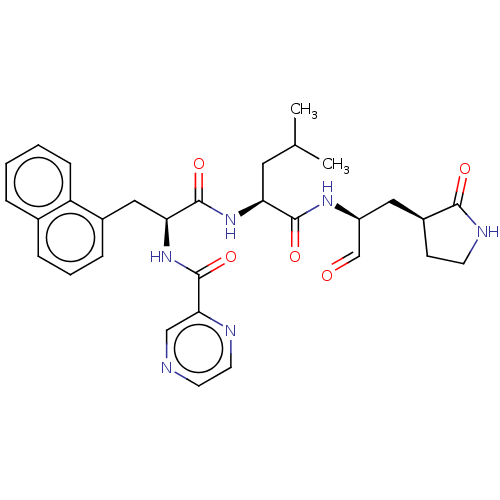
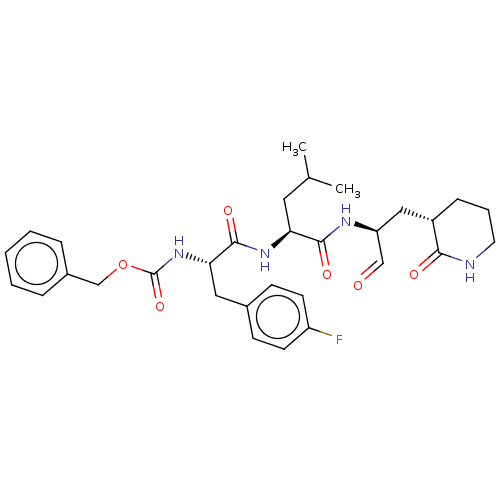
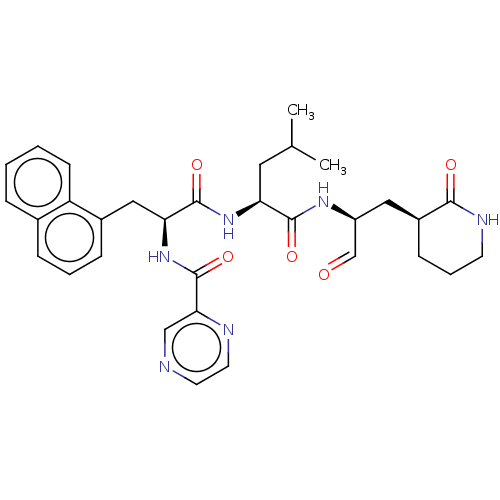
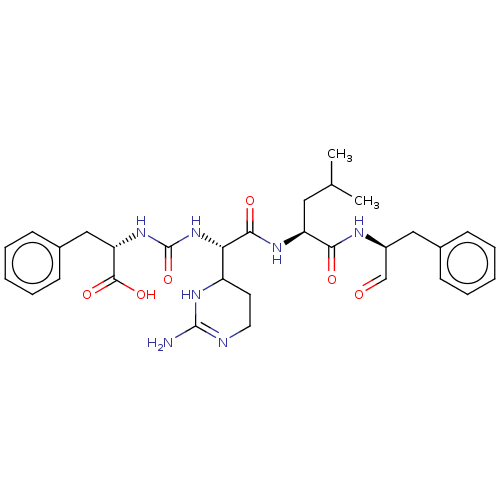
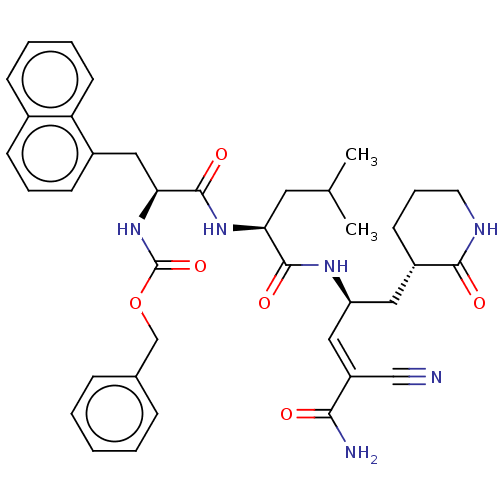
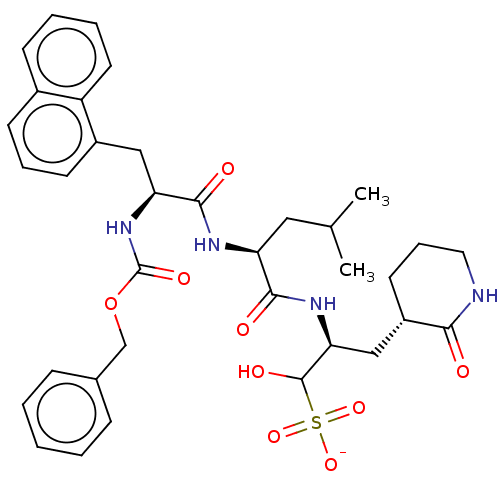
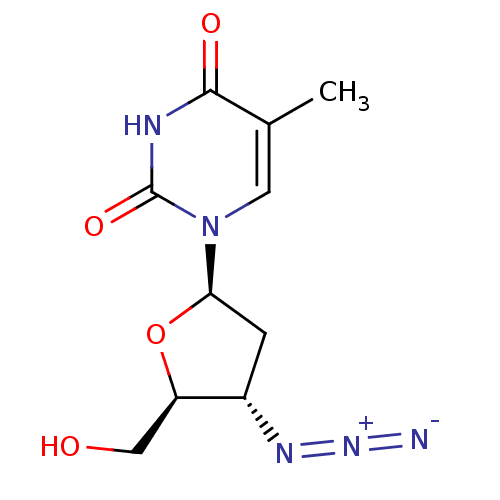
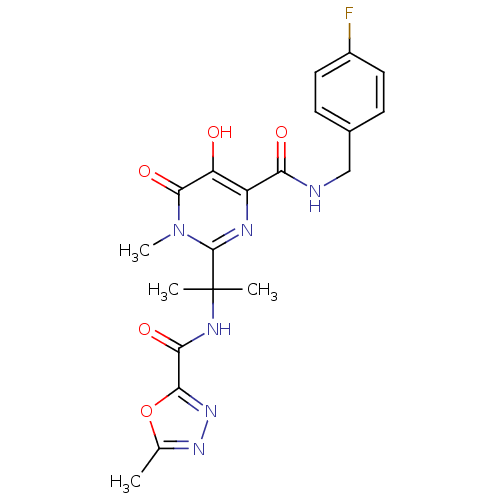
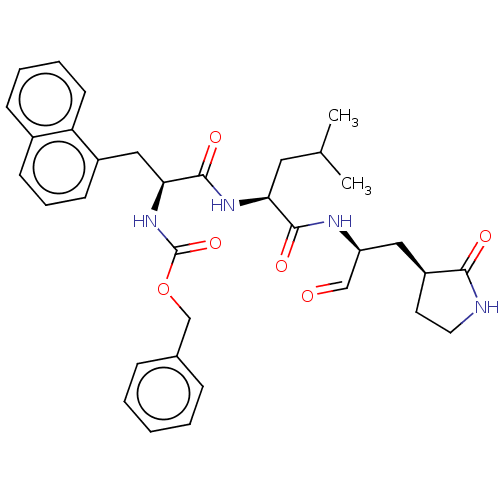
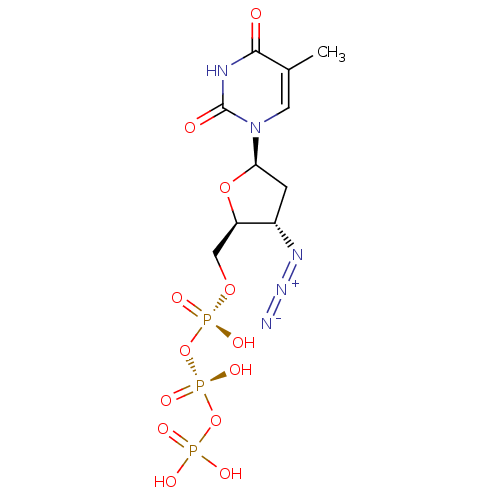
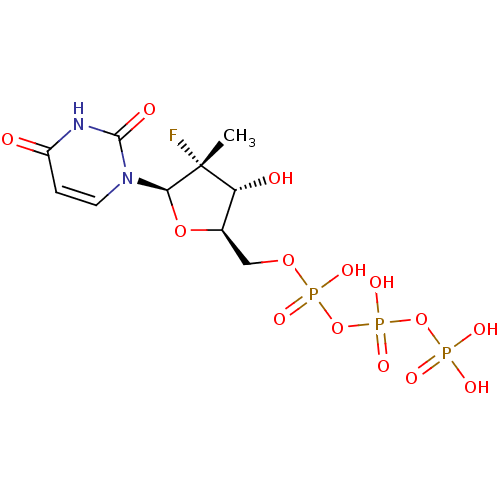
Affinity DataKi: 123nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
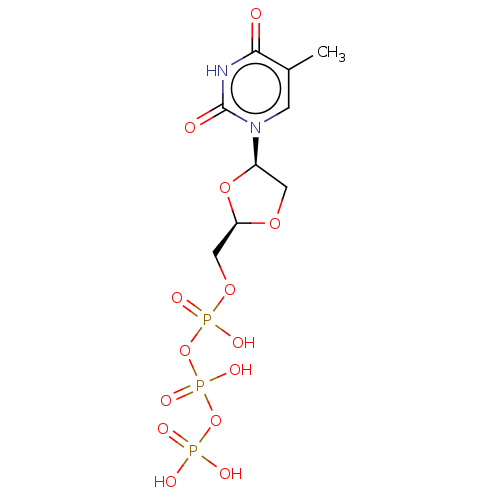
Affinity DataKi: 155nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 256nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 427nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 465nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 528nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 670nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 858nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 1.19E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 1.59E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 2.24E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 7.10E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 8.20E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Tested for the inhibition of the compound towards HIV-reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant HIV-1 integrase stand transfer activity expressed in Escherichia coli BL21(DE3) cells using 32P-labeled DNA substrate after...More data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
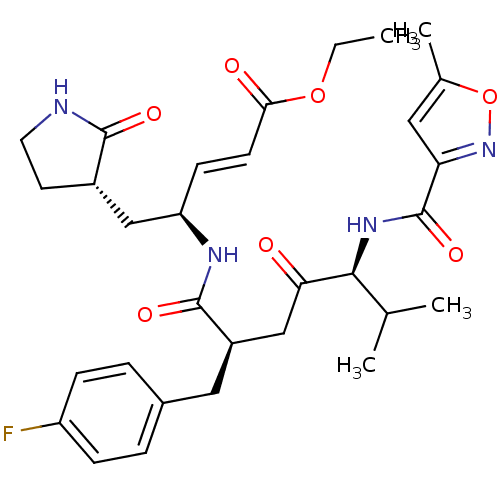
Affinity DataIC50: 25nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of Norovirus prototypic GI.1 3CL protease using HiLyte Fluor 488-labelled DFELQGPK as substrate incubated for 90 mins measured every mins ...More data for this Ligand-Target Pair
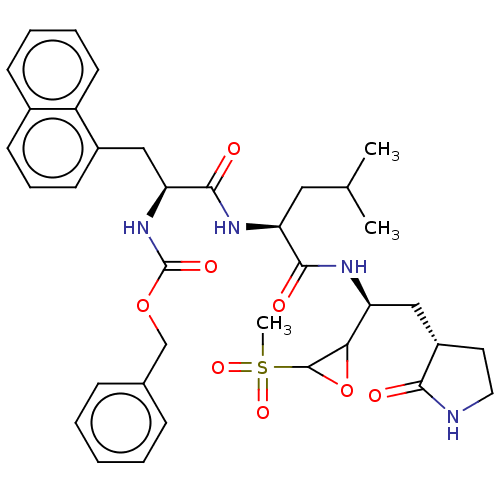
Affinity DataIC50: 44nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 3a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 3a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetGenome polyprotein(Norovirus Hu/GII.4-2002/WeertE022/2002/NL)
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of Norovirus prototypic GII.4 3CL protease using HiLyte Fluor 488-labelled DFELQGPK as substrate incubated for 90 mins measured every mins...More data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 1b infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 1b infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 4a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 4a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of Norovirus prototypic GI.1 3CL protease using HiLyte Fluor 488-labelled DFELQGPK as substrate incubated for 90 mins measured every mins ...More data for this Ligand-Target Pair
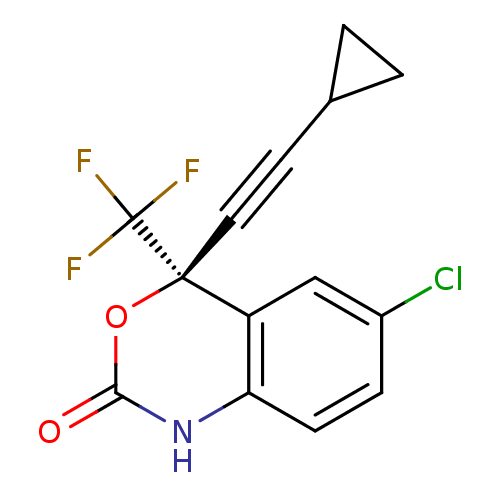
Affinity DataIC50: 80nMAssay Description:Inhibition of HIV1 LAI reverse transcriptase wild type LAI by heteropolymeric DNA polymerase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 84nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 96nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 96nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 96nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataIC50: 96nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 5a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
TargetRNA-directed RNA polymerase(Hepatitis C virus)
Emory University School Of Medicine
Curated by ChEMBL
Emory University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of NS5B polymerase in HCV genotype 5a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of HIV1 pNL4-3 reverse transcriptase wild type pNL4-3 by heteropolymeric DNA polymerase assayMore data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:Inhibition of HIV-1 reverse transcriptase from peripheral blood mononuclear cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of HIV1 LAI reverse transcriptase wild type LAI by heteropolymeric DNA polymerase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair