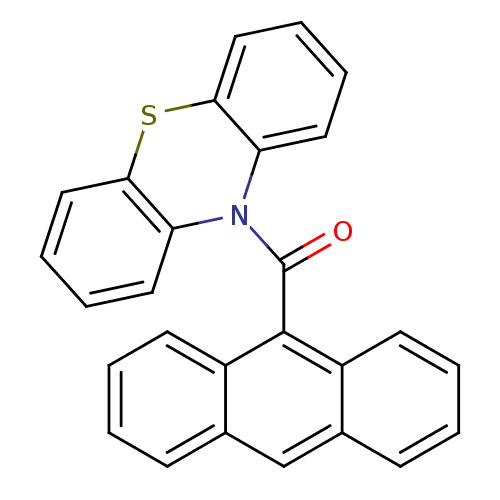
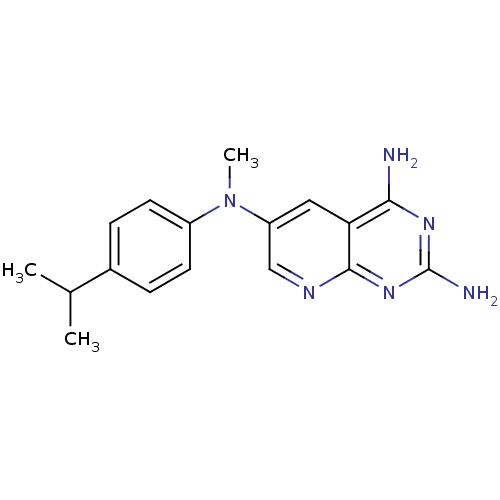
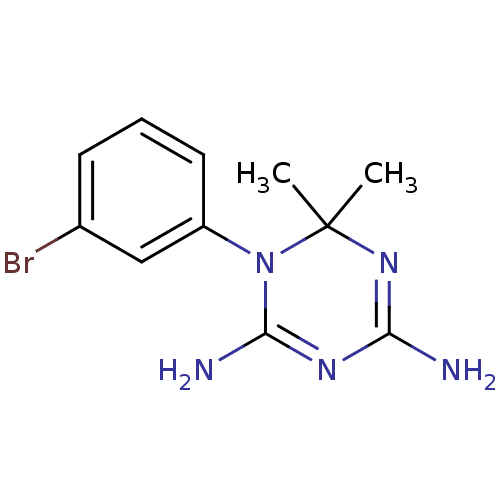
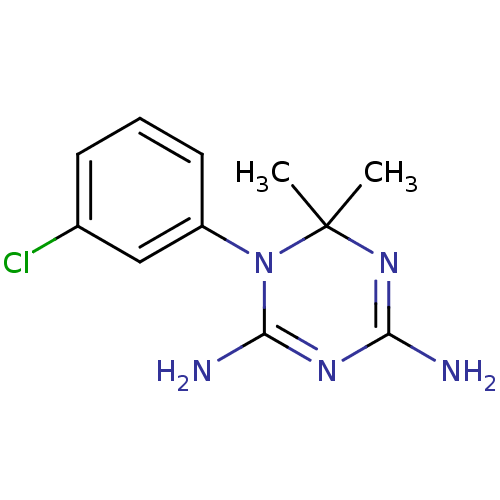
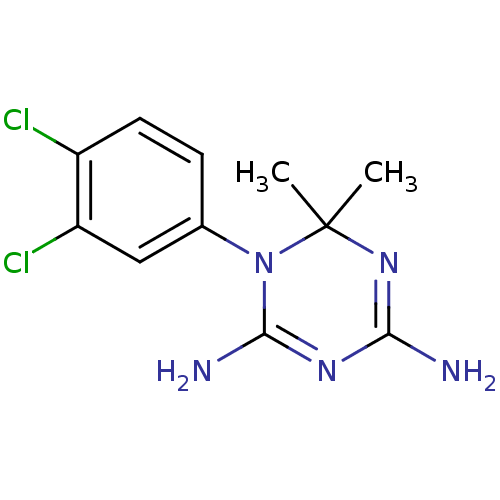
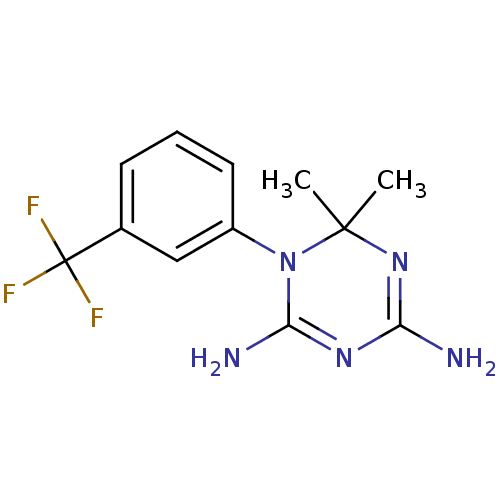
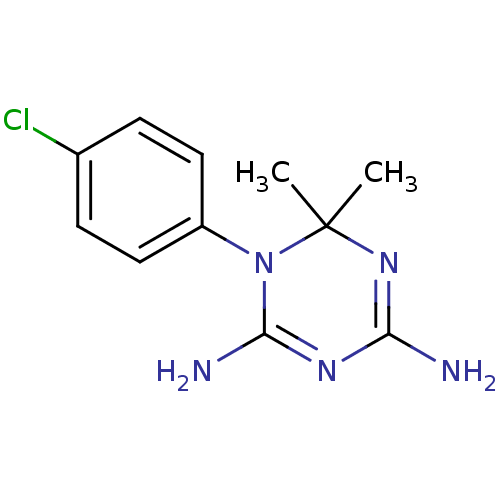
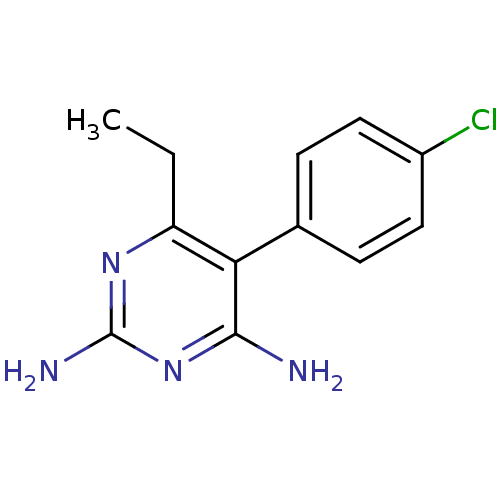
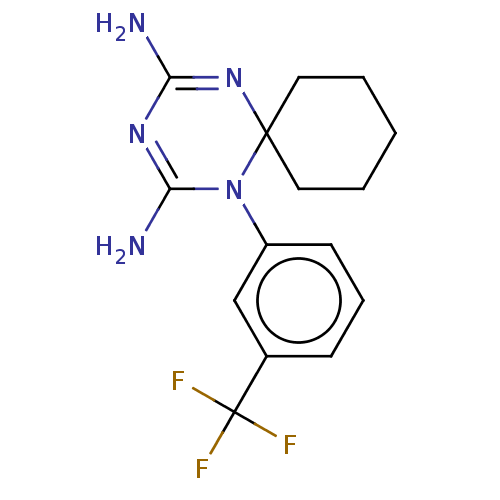
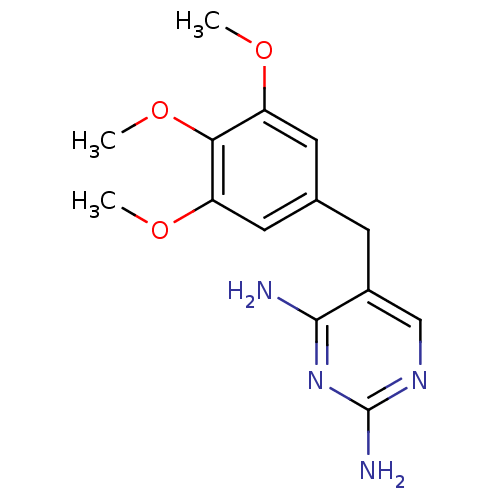
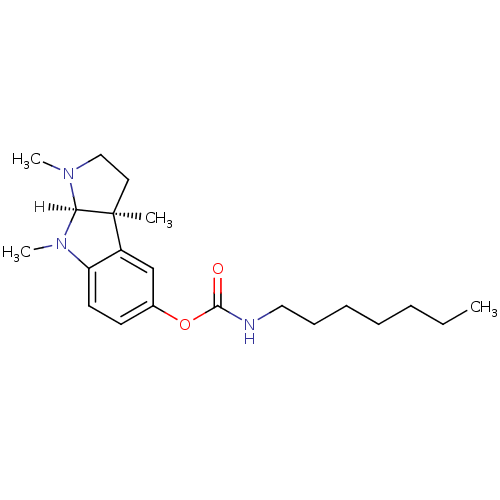
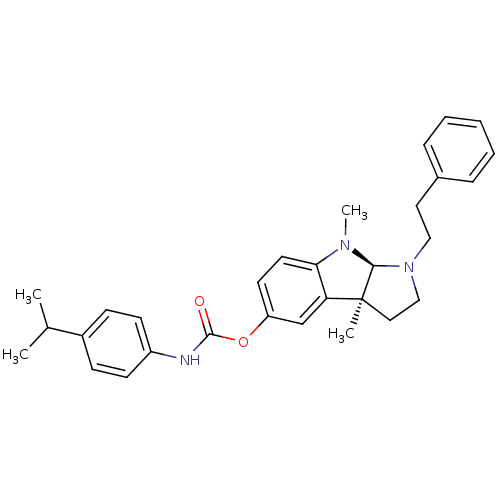
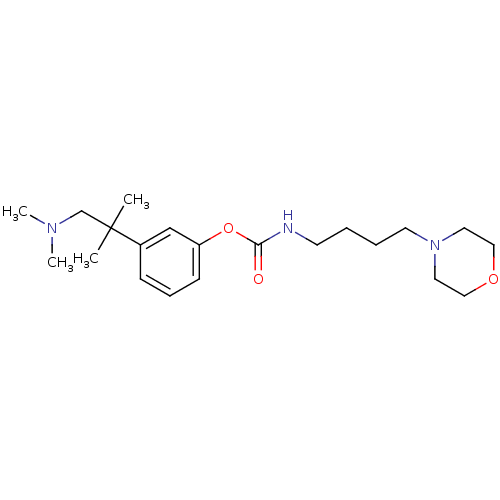
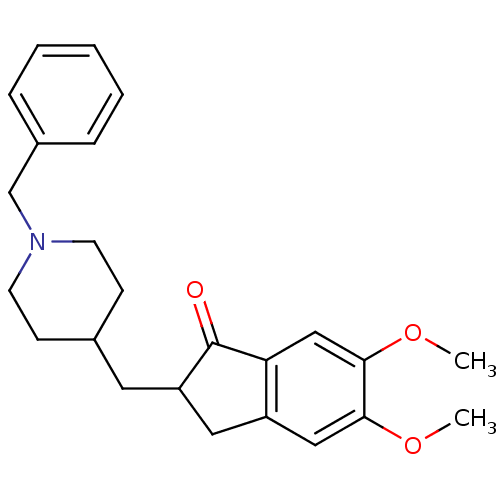
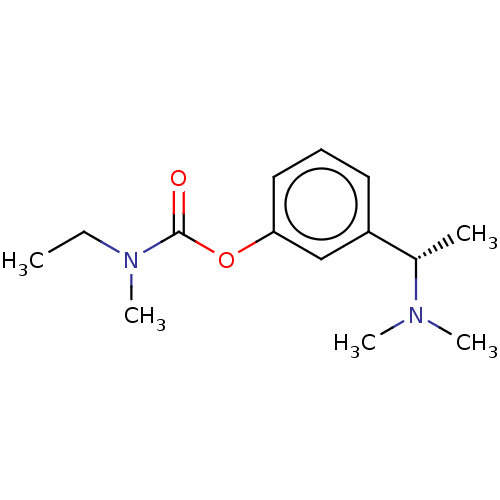
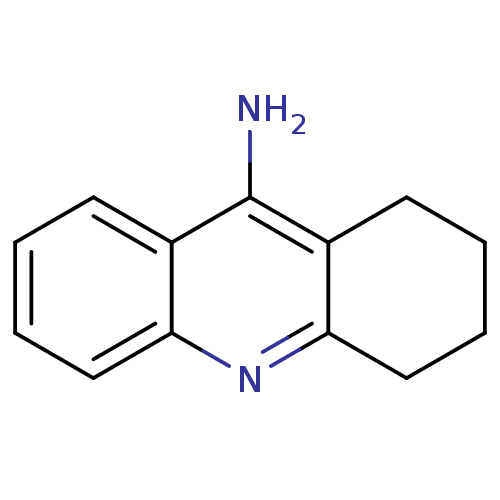
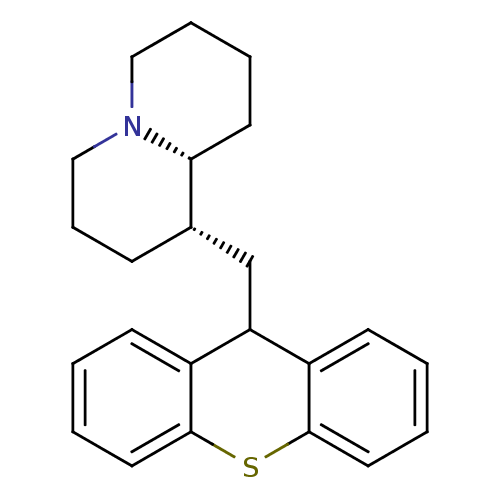
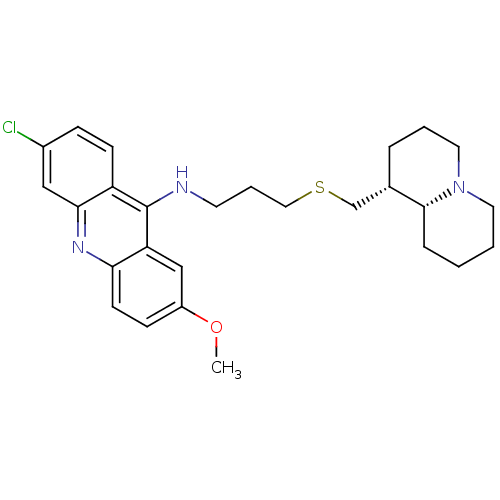
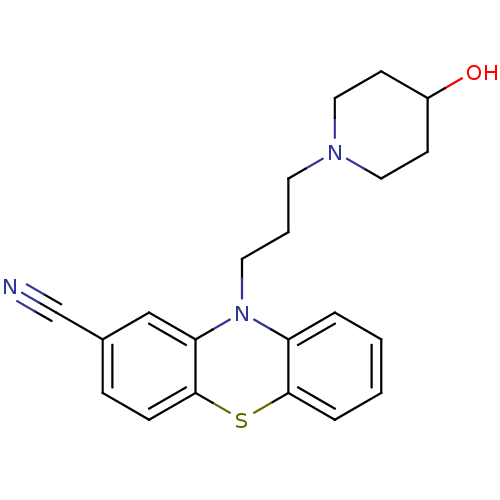
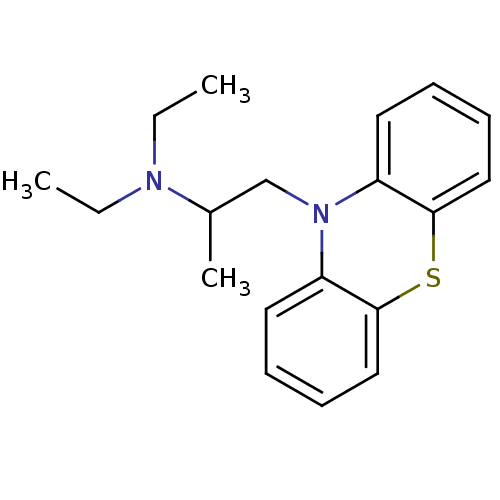
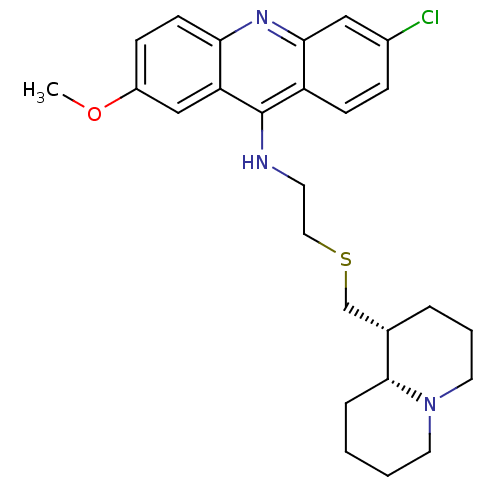
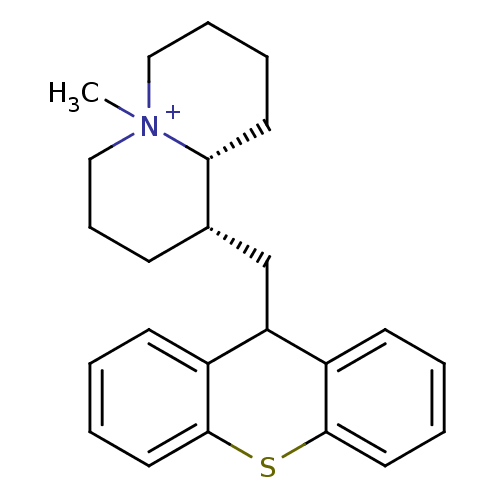
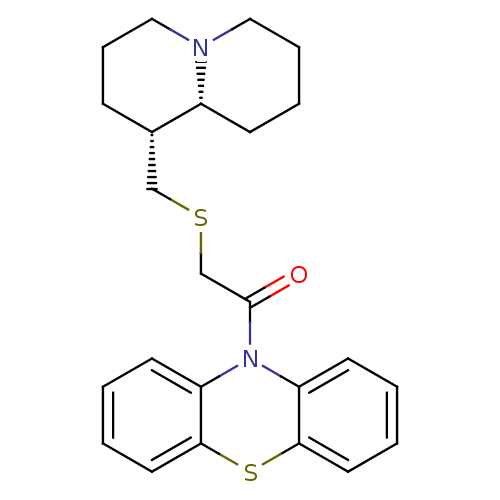
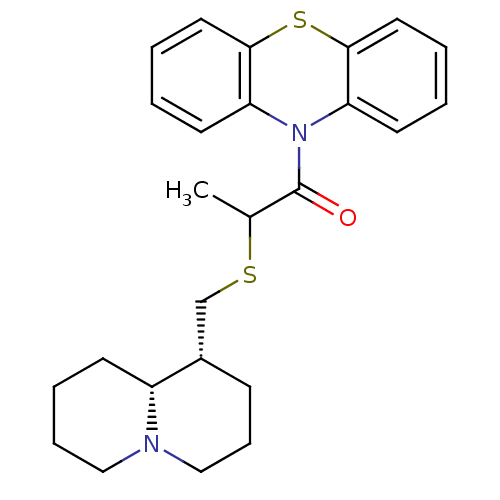
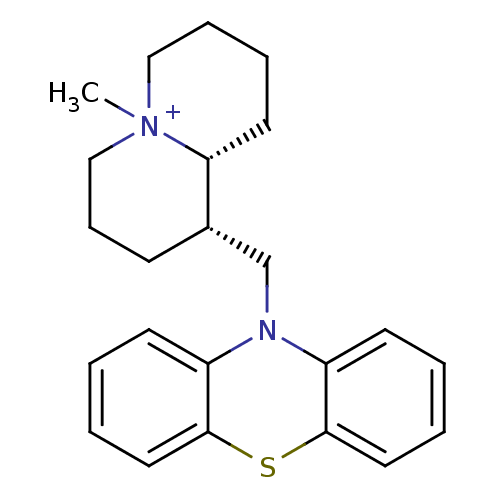
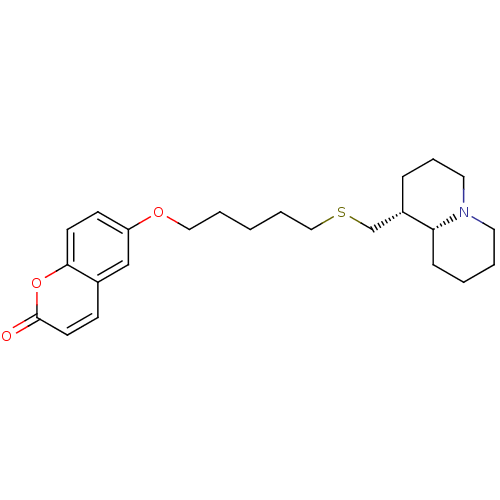
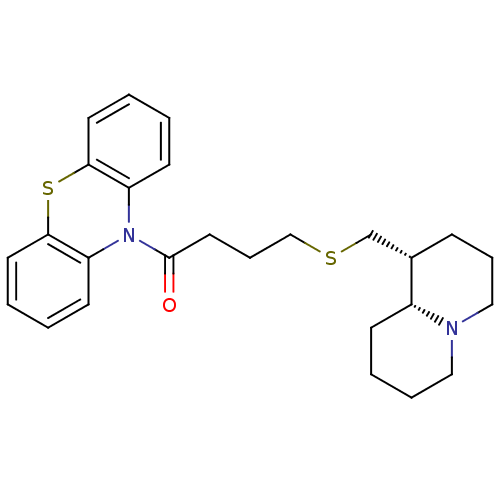
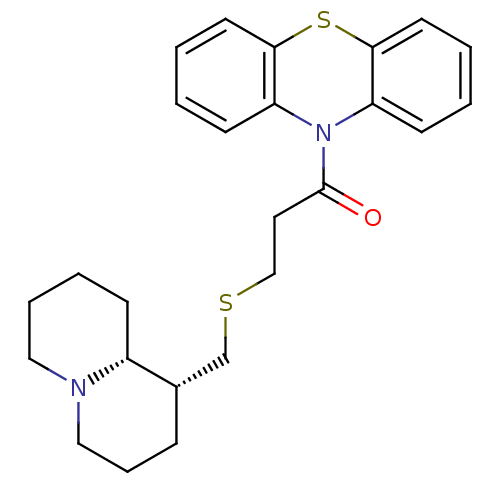
Affinity DataKi: 0.0100nMAssay Description:Inhibition of DHFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 470nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 530nMAssay Description:Binding affinity to human recombinant DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 640nMAssay Description:Binding affinity to human recombinant DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Binding affinity to human recombinant DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.32E+4nMAssay Description:Inhibition of human DHFR using dihydrofolate as substrate after 180 secs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.30E+4nMAssay Description:Inhibition of human DHFRMore data for this Ligand-Target Pair
Affinity DataKi: 4.80E+4nMAssay Description:Inhibition of human DHFRMore data for this Ligand-Target Pair
Affinity DataKi: 1.09E+5nMAssay Description:Inhibition of Pneumocystis carinii DHFRMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+5nMAssay Description:Inhibition of Pneumocystis carinii DHFRMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human erythrocytes AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human plasma AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human erythrocytes AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of human plasma AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of human erythrocytes AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of bovine erythrocyte AChE using acetylthiocholine iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Inhibition of human erythrocytes AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 630nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Inhibition of bovine erythrocyte AChE using acetylthiocholine iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 740nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair

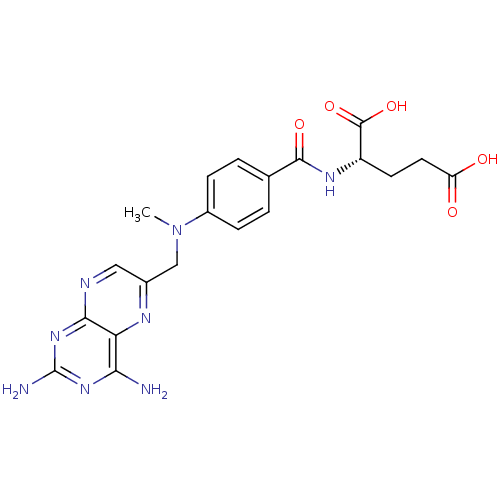
 3D Structure (crystal)
3D Structure (crystal)