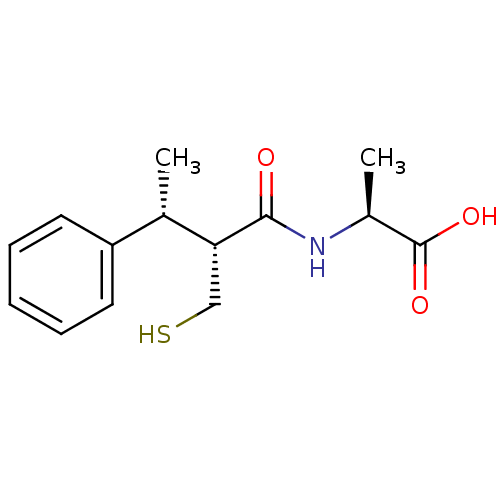
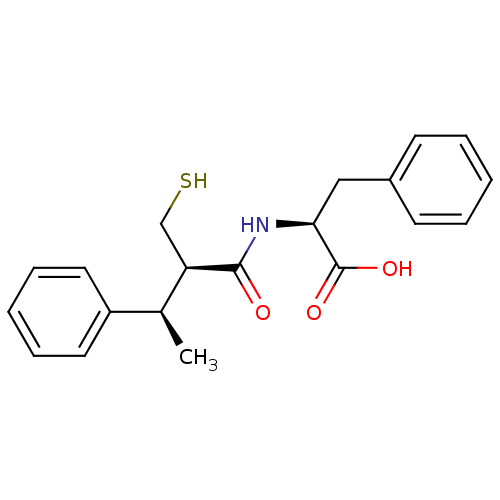
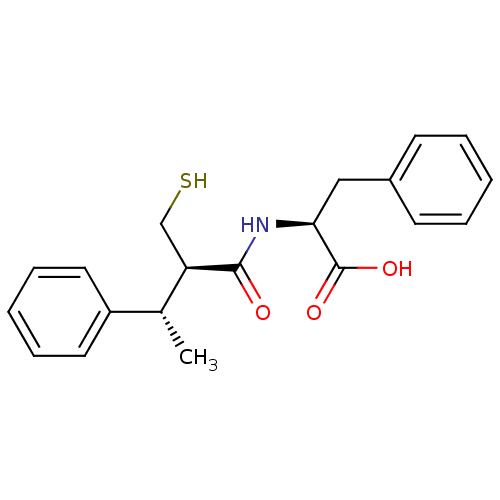
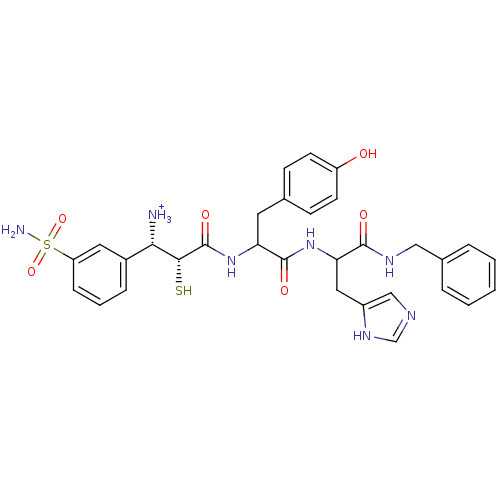
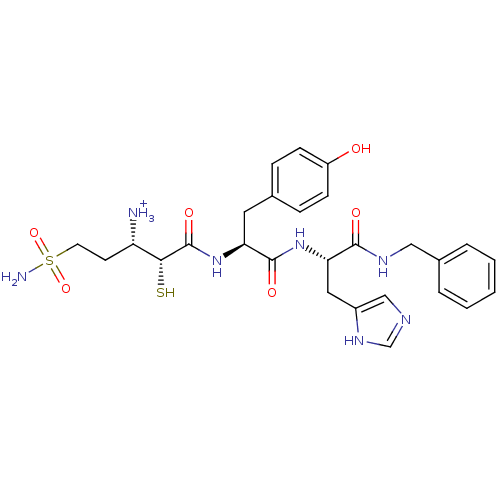
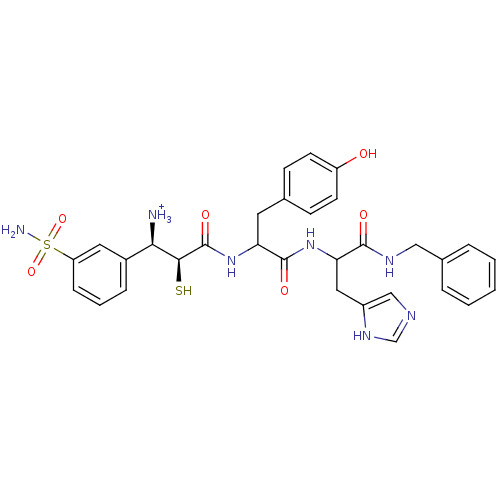
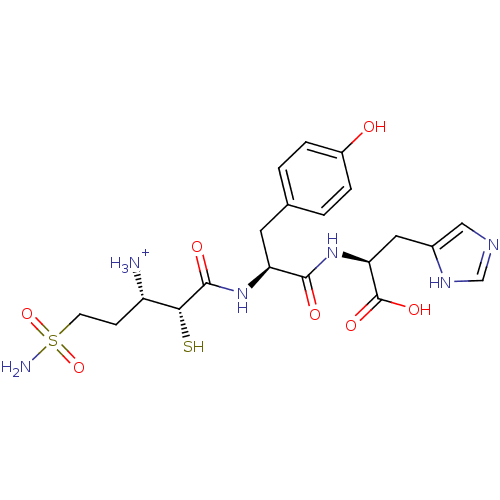
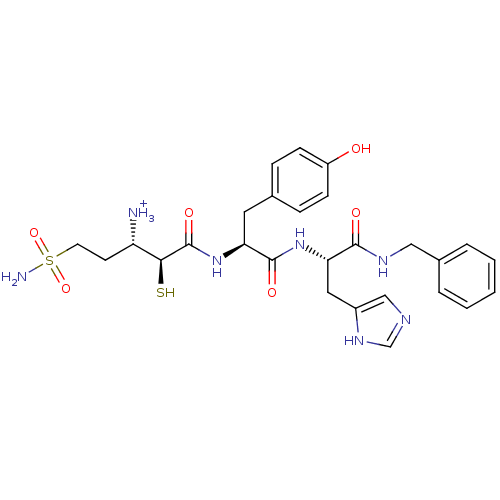
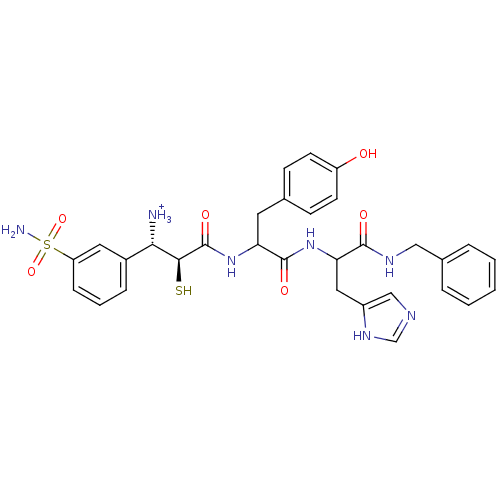
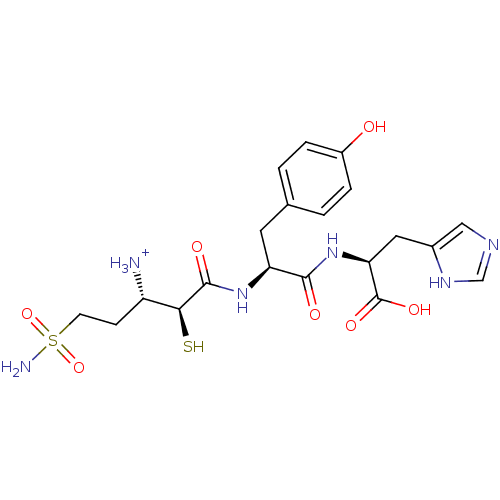
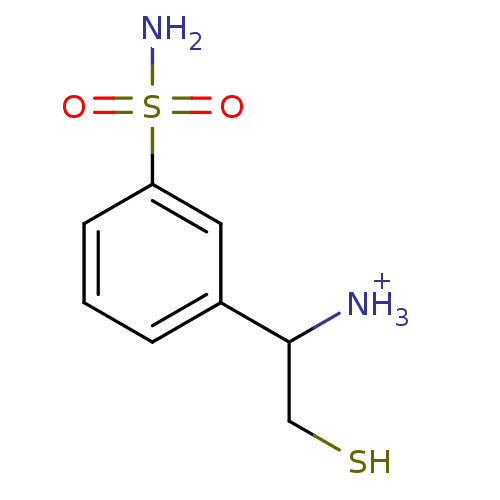
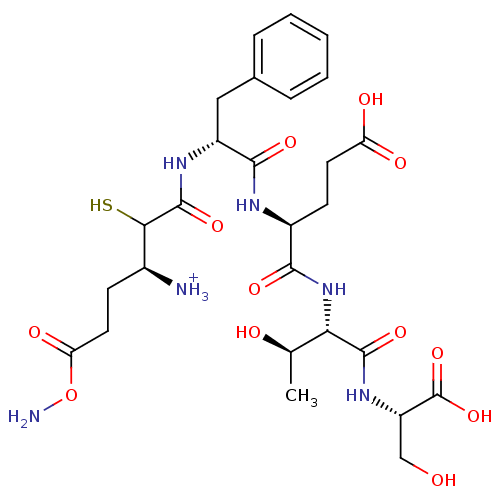
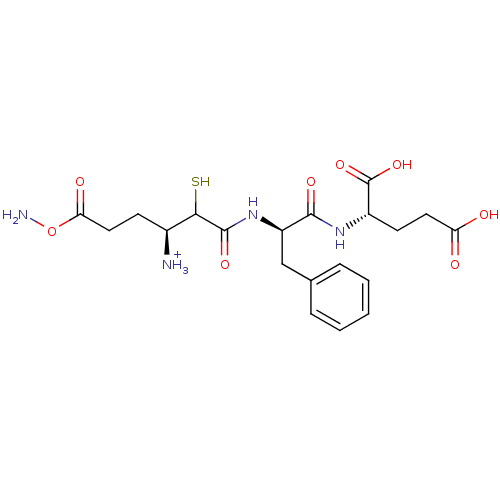
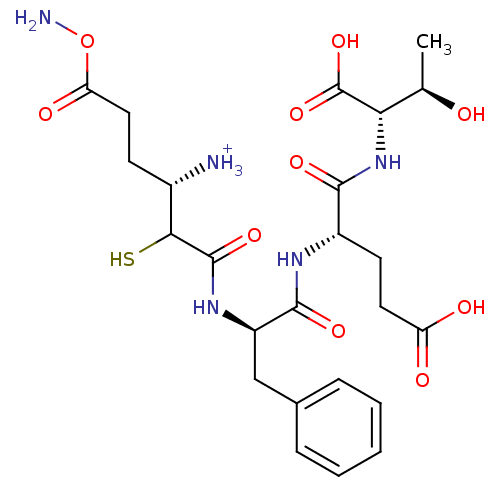
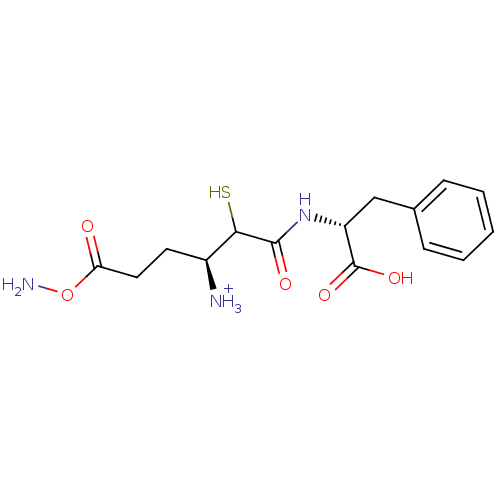
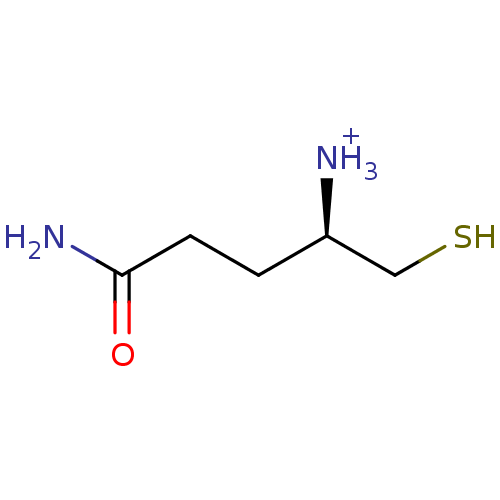
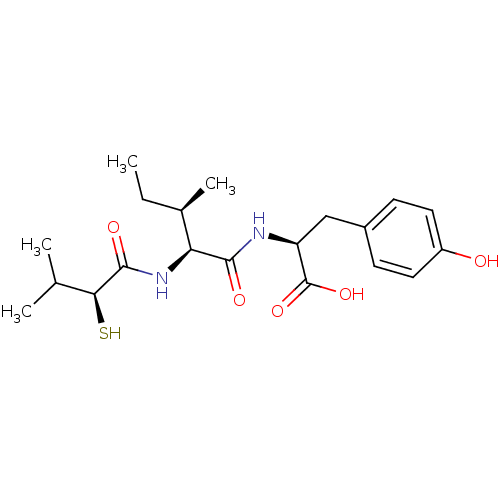
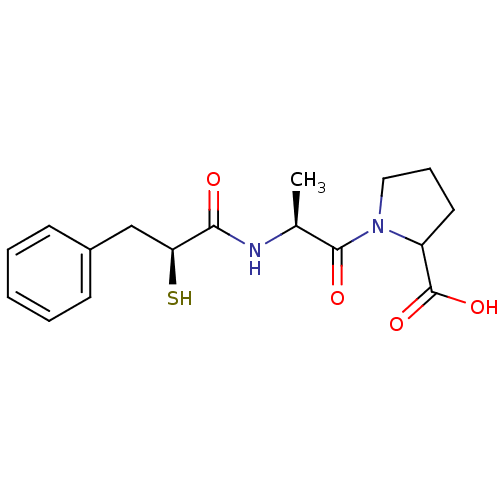
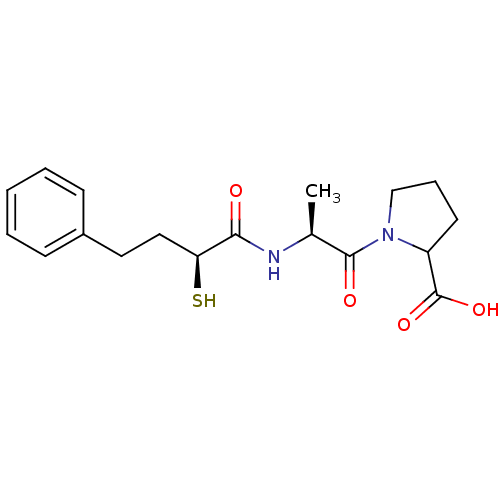
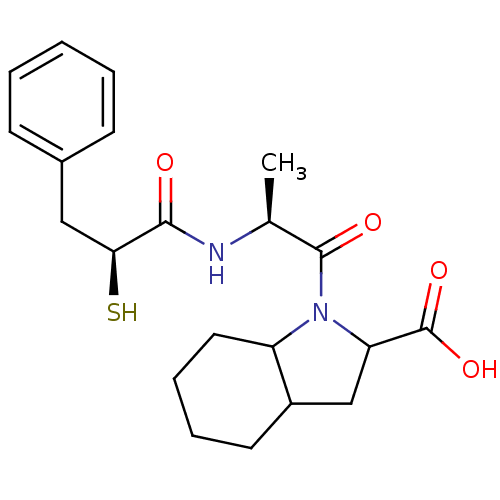
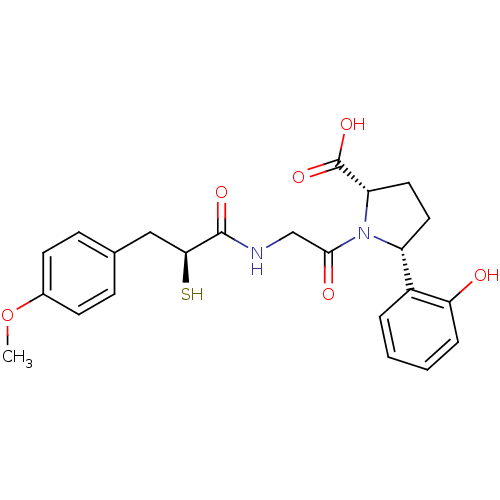
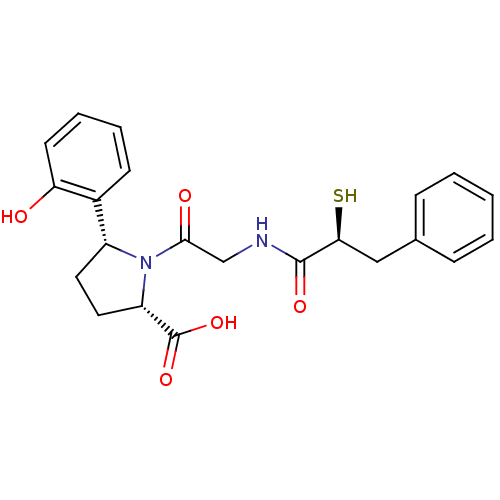
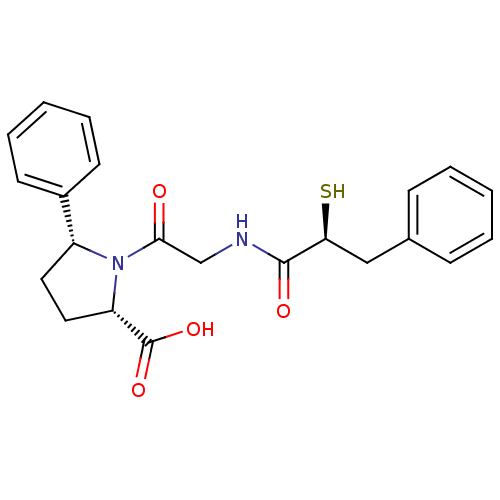
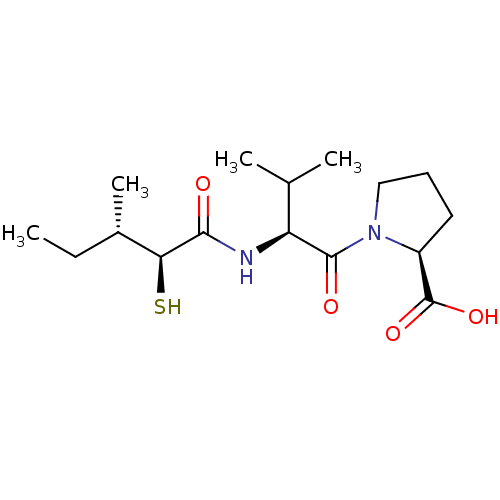
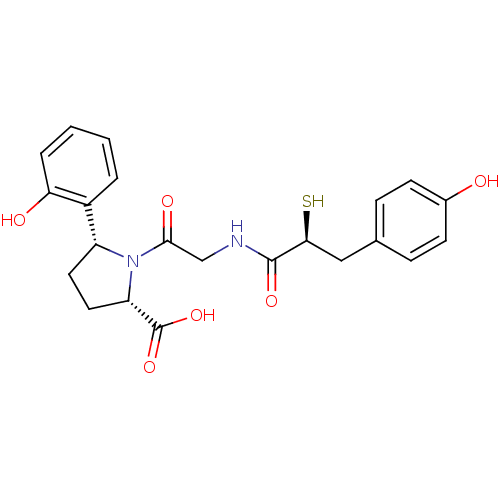
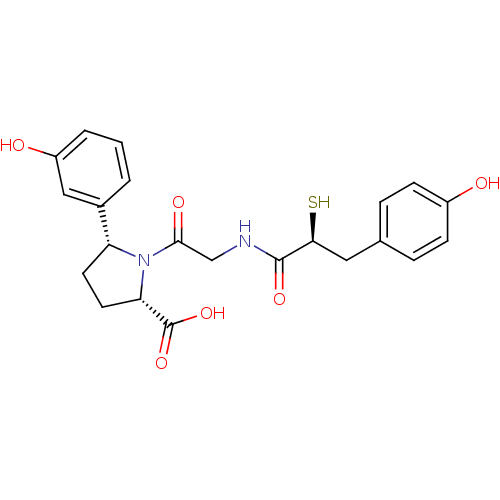
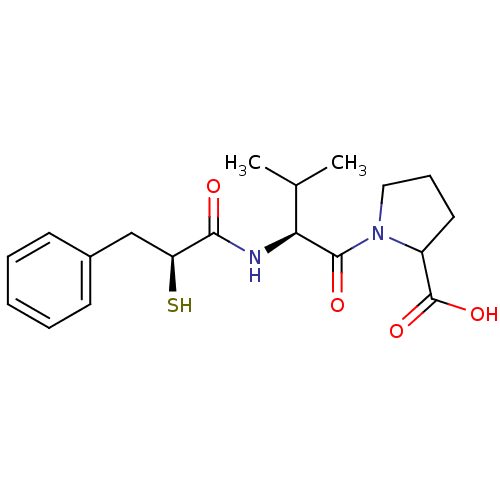
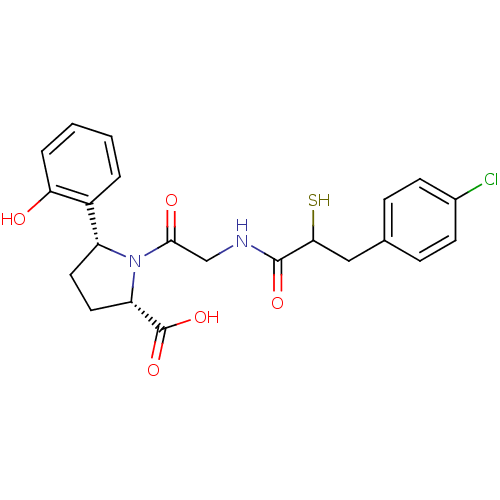
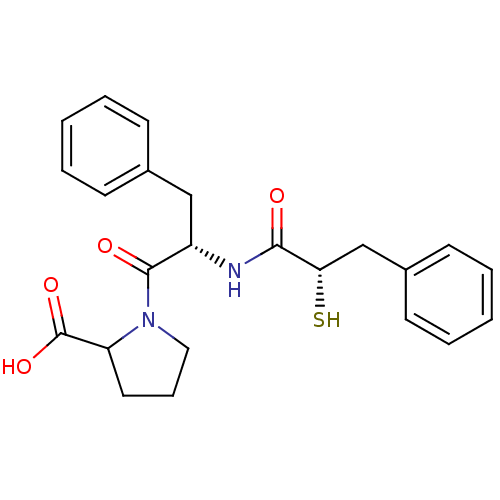
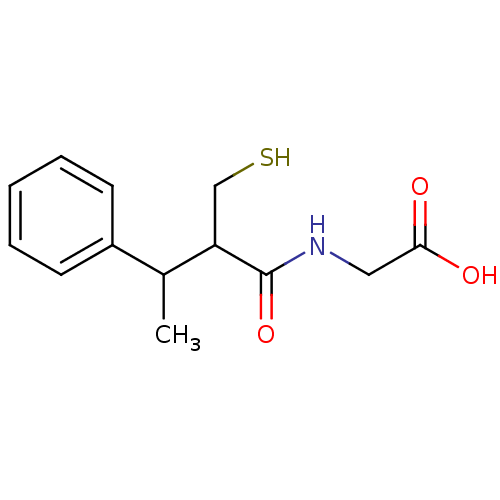
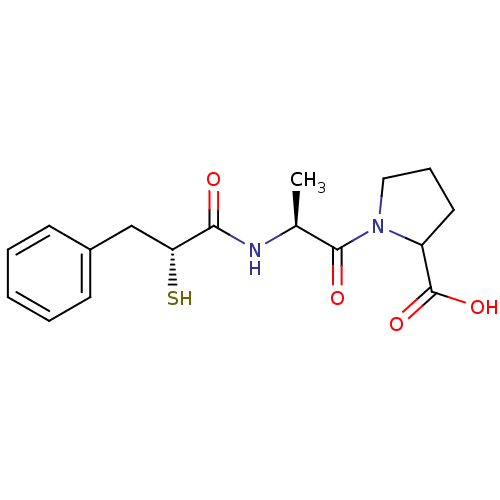
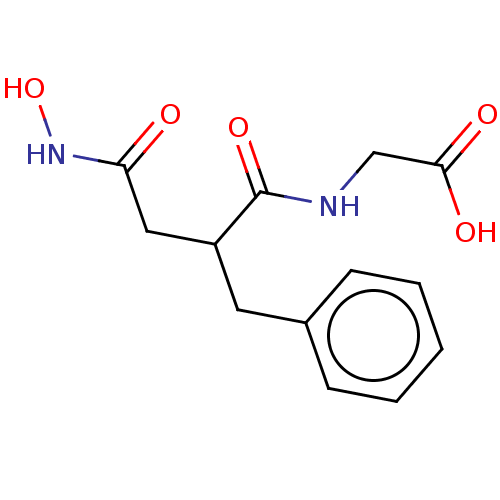
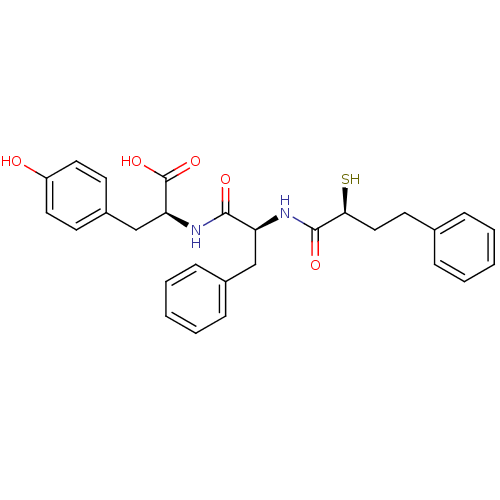
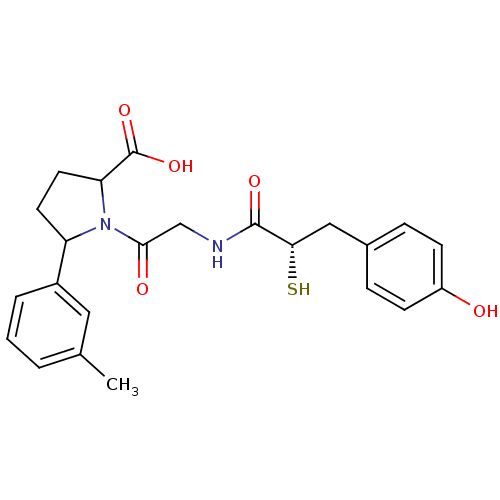
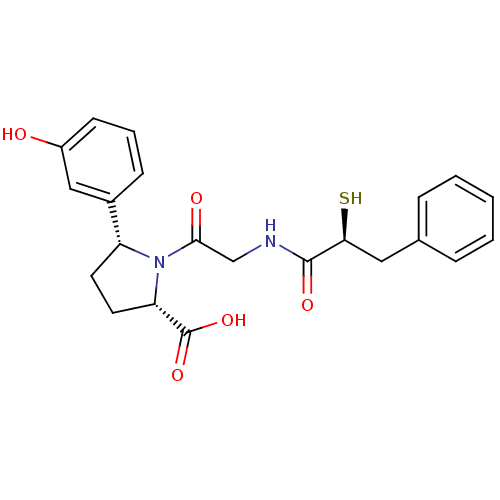
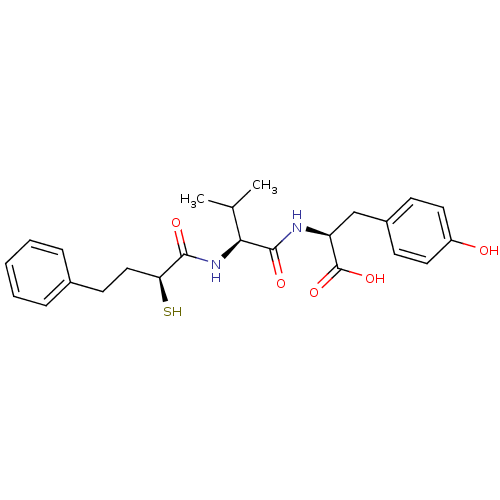
Affinity DataKi: 0.700nMAssay Description:In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lungMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lungMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidneyMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lungMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lungMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lungMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 6.80E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 9.60E+3nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 3.50E+4nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+4nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataKi: 2.50E+5nMAssay Description:Inhibition of tetanus neurotoxin (TeNt)More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.350nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In vitro inhibition of neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vitro inhibition of neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidneyMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibitory activity was tested against neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory potency against neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory activity was tested against neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:In vitro inhibition of neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:In vitro inhibition of rat angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:In vitro inhibition of neutral endopeptidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibitory activity against angiotensin I converting enzymeMore data for this Ligand-Target Pair