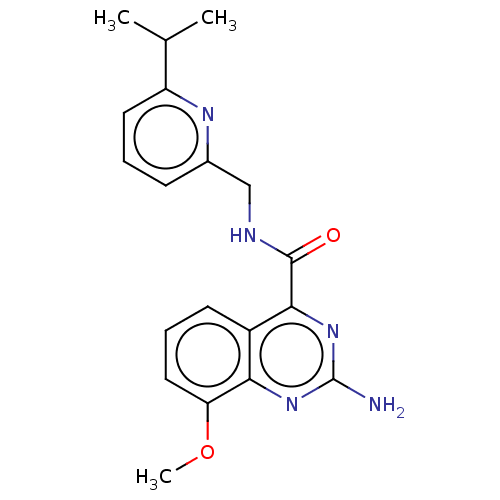
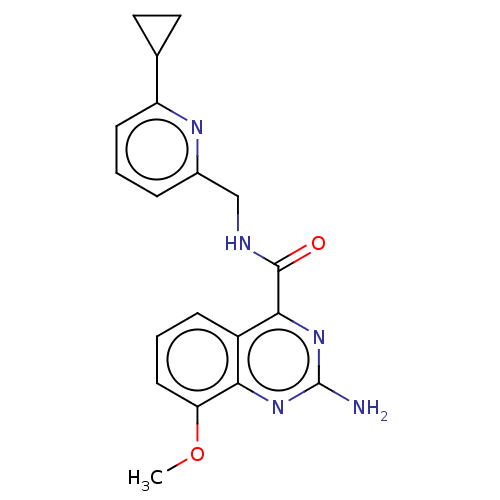
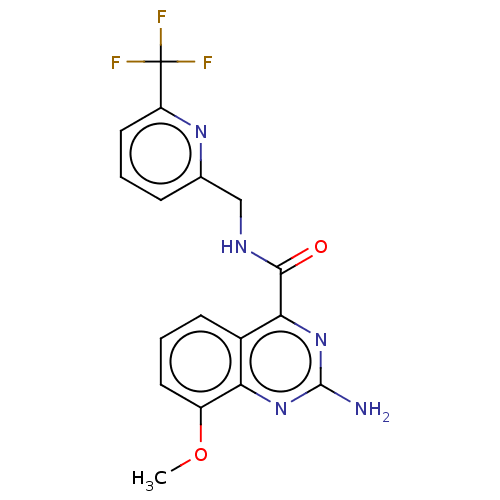
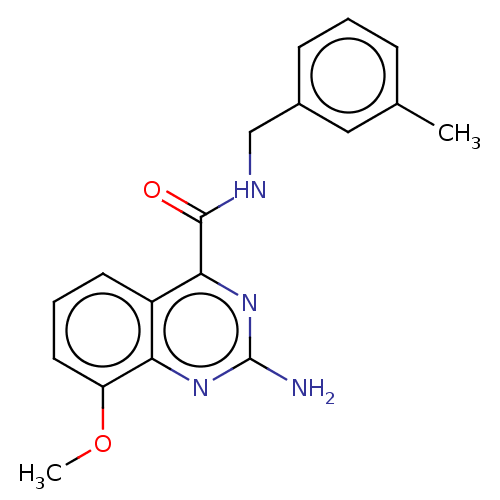
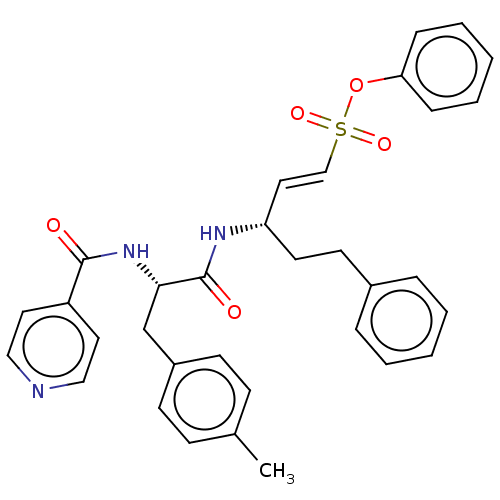
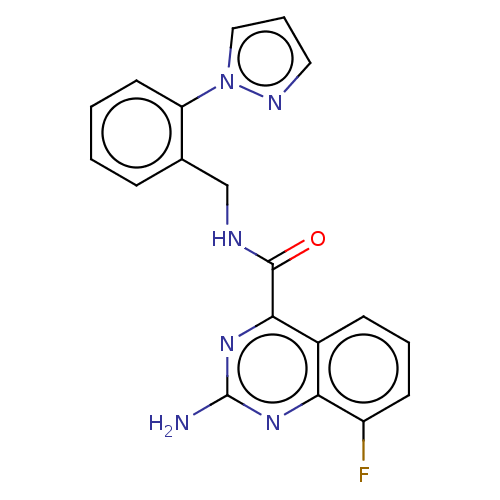
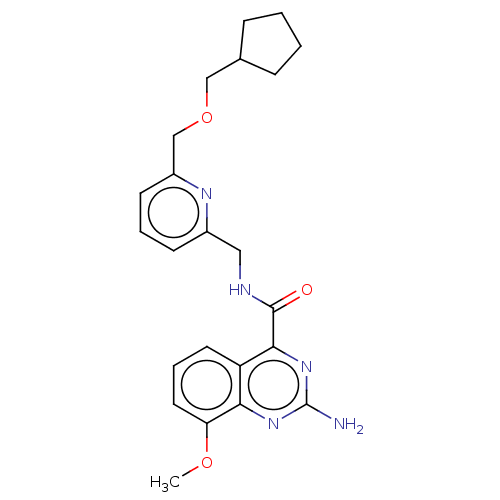
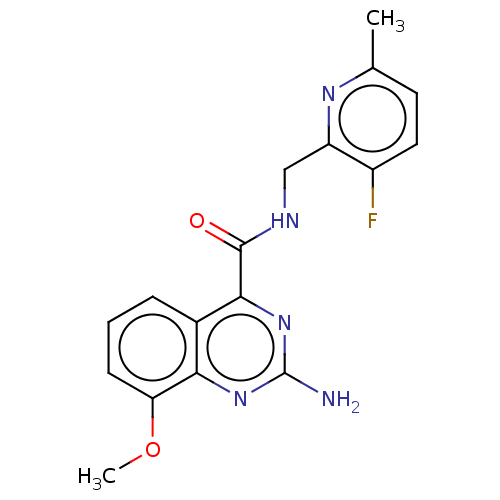
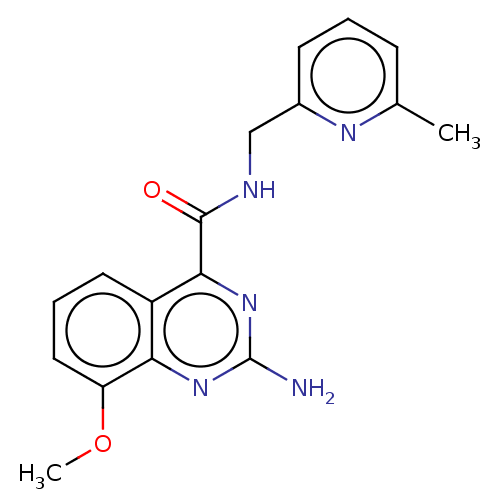
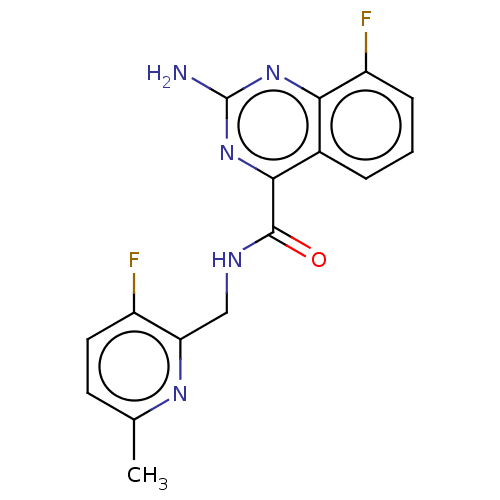
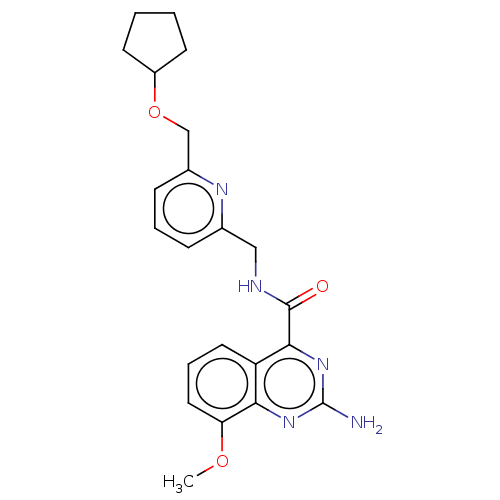
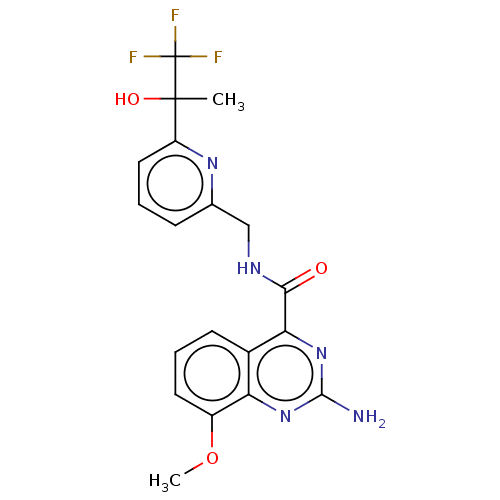
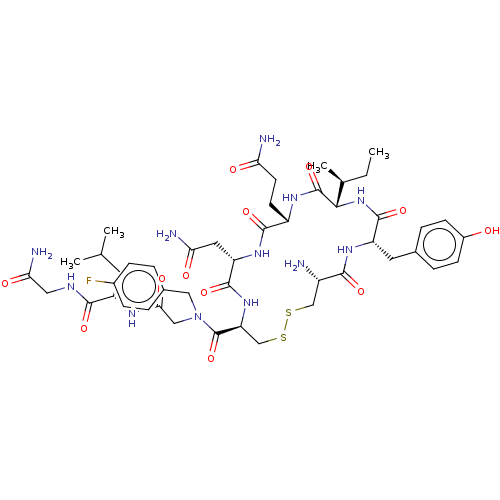
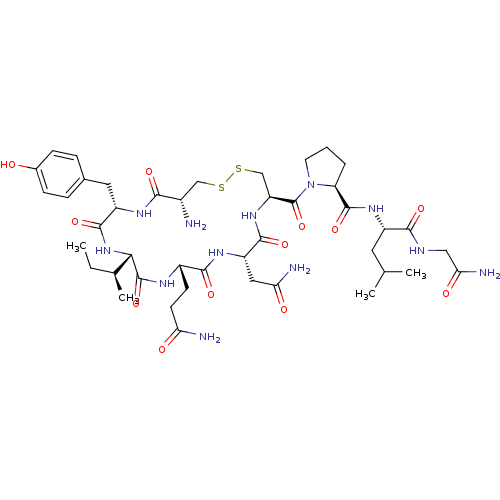
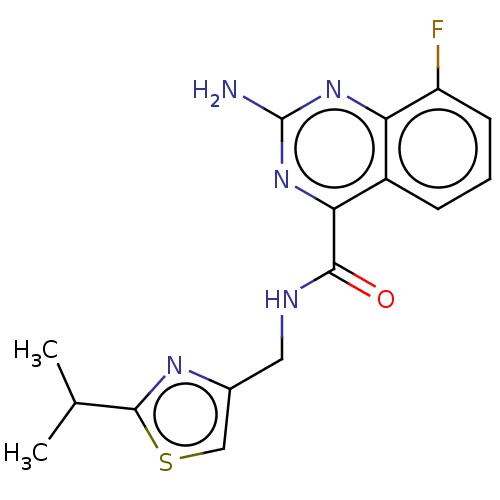
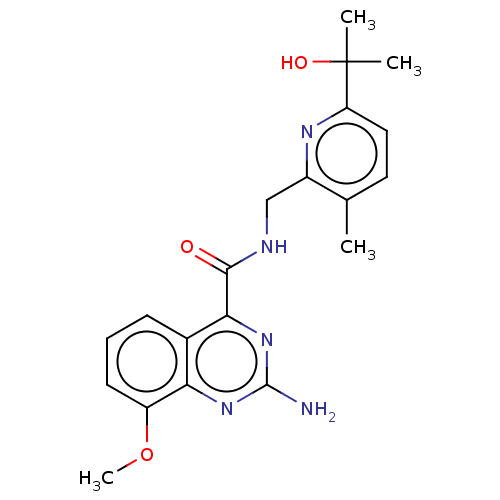
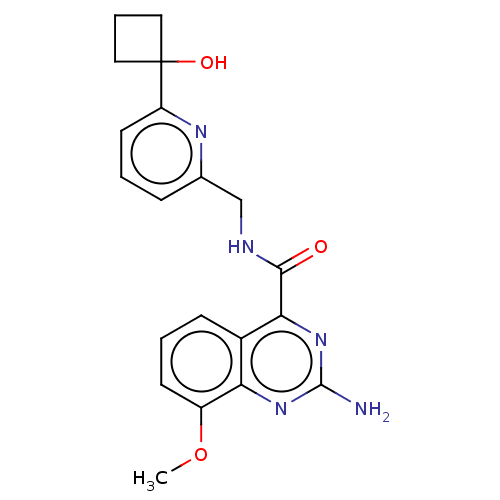
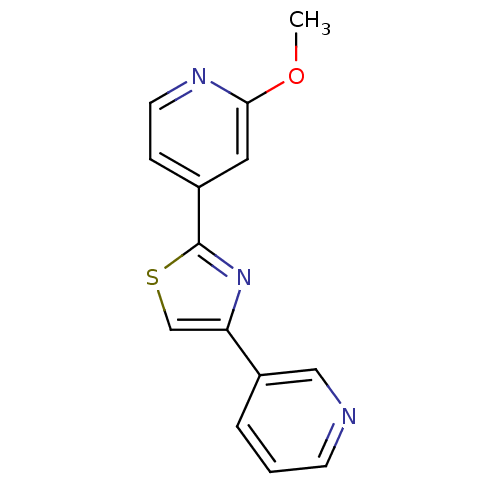
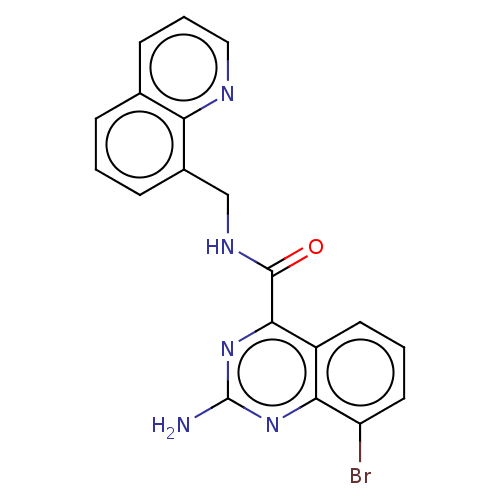
Affinity DataKi: 0.00110nM IC50: 1.00E+3nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
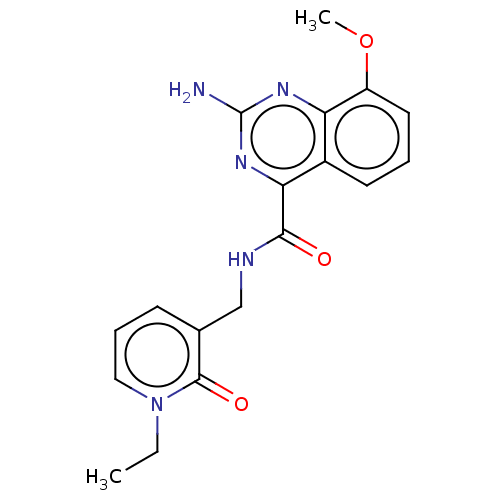
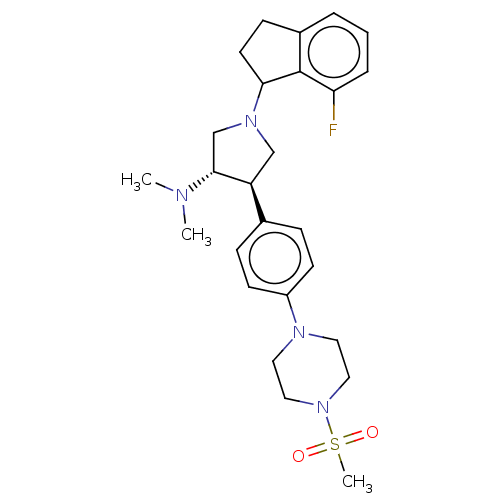
Affinity DataKi: 0.00182nM IC50: 1.21E+4nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
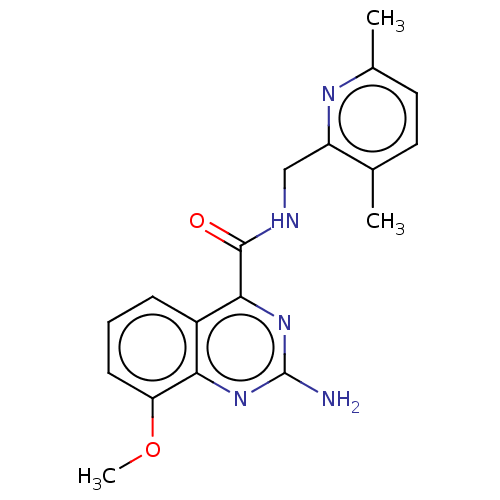
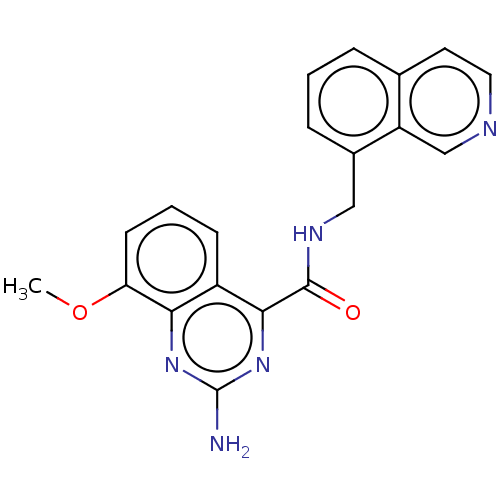
Affinity DataKi: 0.00216nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
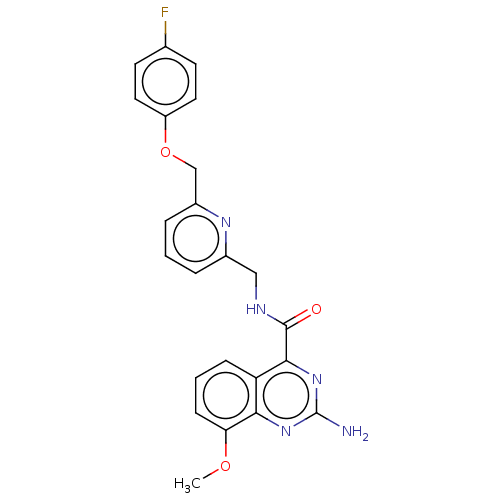
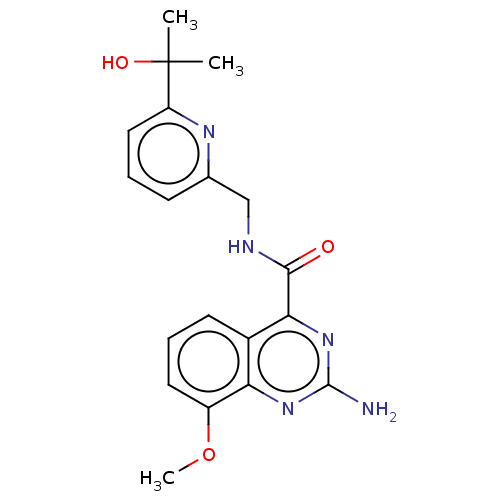
Affinity DataKi: 0.00256nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00425nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00597nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.00838nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.0118nMAssay Description:Inhibition assay using aromatase enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetSigma non-opioid intracellular receptor 1(Cavia porcellus (Guinea pig))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataKi: 0.0300nMAssay Description:Displacement of [3H]-pentazocine from sigma 1 receptor in guinea pig brain membrane after 120 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetSigma non-opioid intracellular receptor 1(Cavia porcellus (Guinea pig))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H]-pentazocine from sigma 1 receptor in guinea pig brain membrane after 120 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
TargetPolycomb protein EED(Homo sapiens (Human))
Sanquan College Of Xinxiang Medical University
Curated by ChEMBL
Sanquan College Of Xinxiang Medical University
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Binding affinity to GST-tagged EED (unknown origin) assessed as inhibition constant incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Johannes Gutenberg University
Curated by ChEMBL
Johannes Gutenberg University
Curated by ChEMBL
Affinity DataKi: 0.450nMAssay Description:Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.510nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.580nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinity to rat A2A adenosine receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an...More data for this Ligand-Target Pair

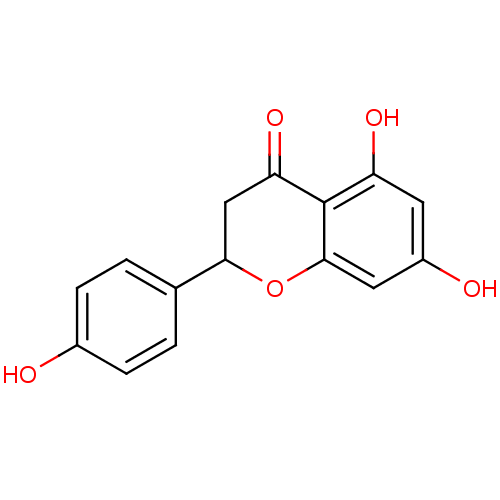
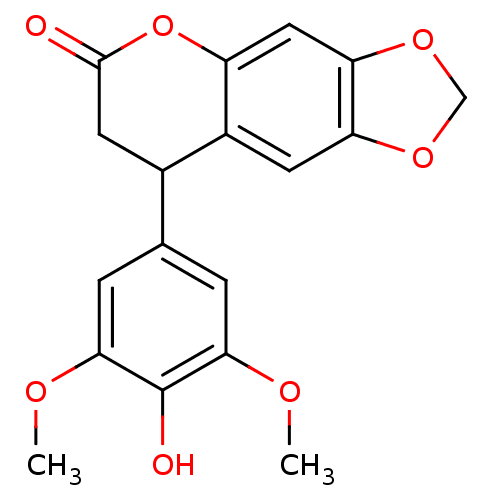
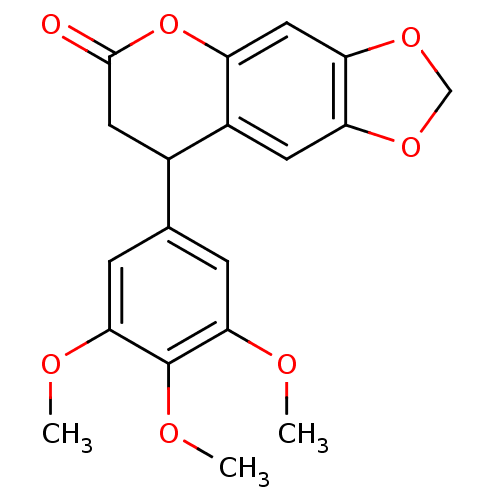
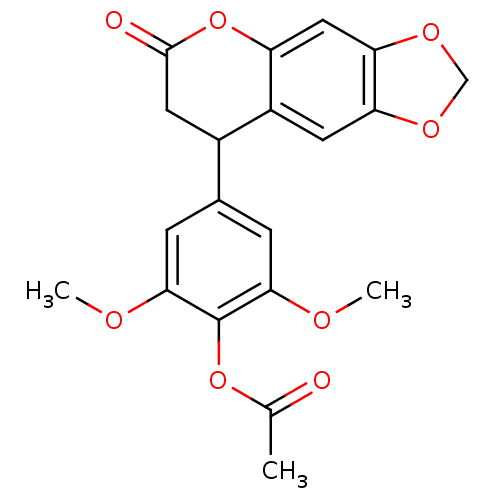
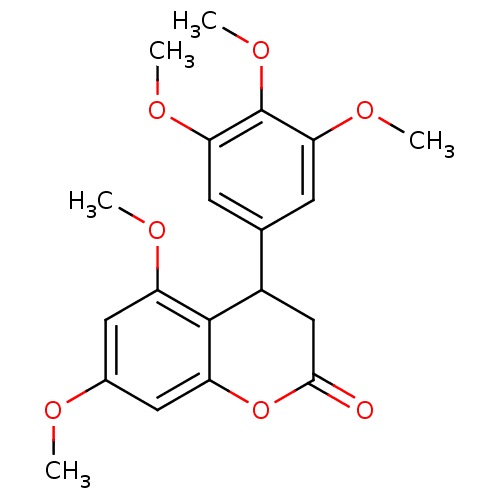
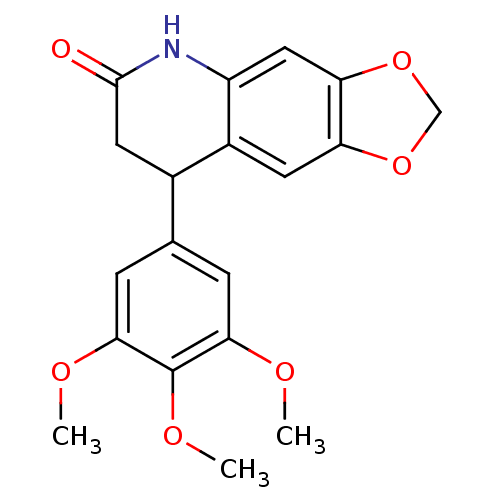
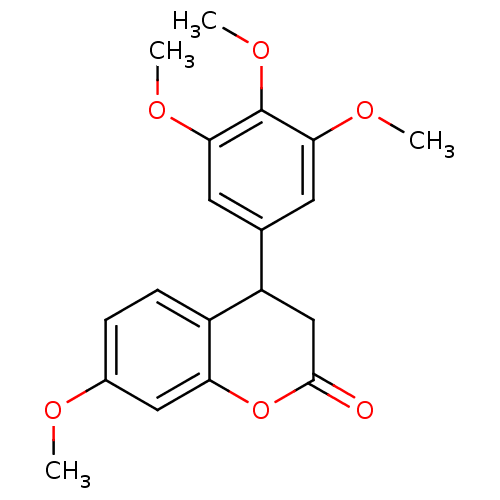


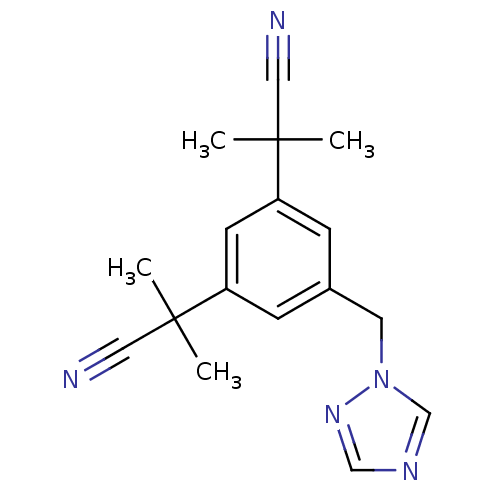
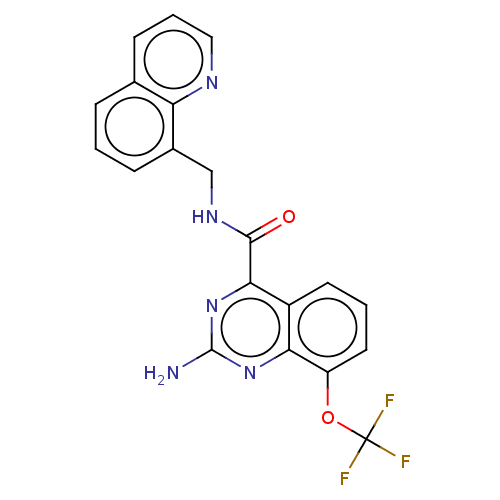
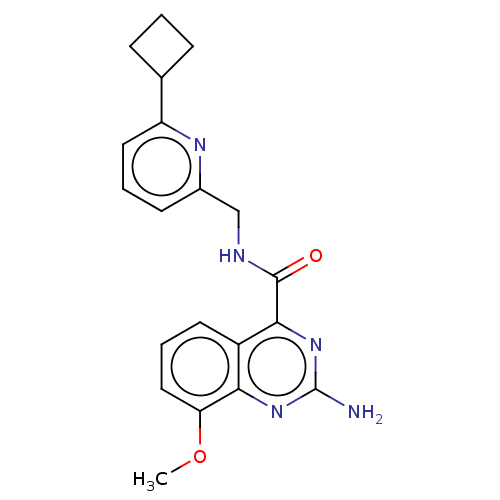
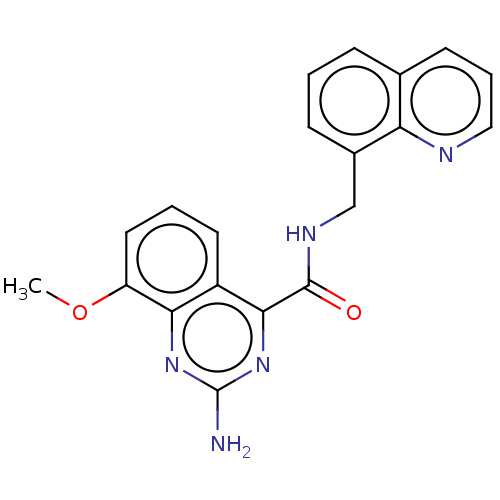
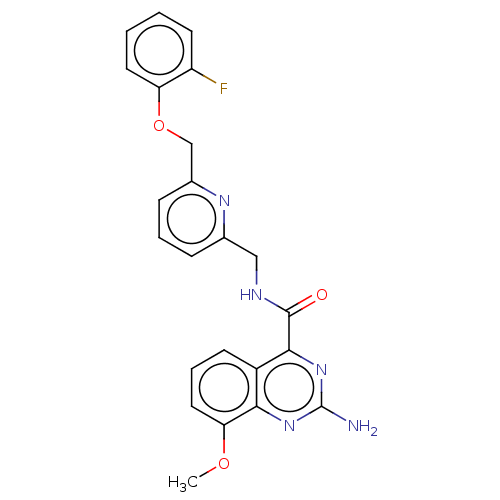
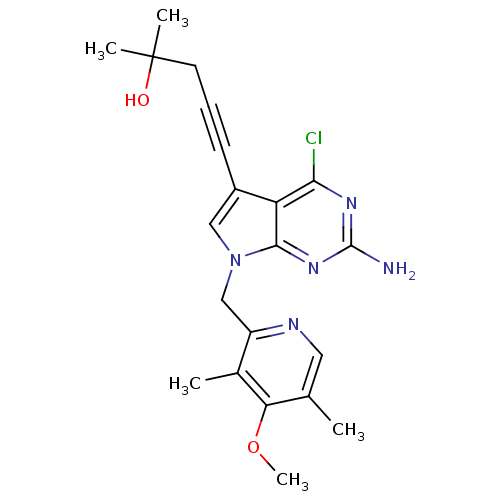
 3D Structure (crystal)
3D Structure (crystal)